Amines, Amides and Amino Acids Flashcards
1/6
Earn XP
Description and Tags
Made on the 17/01/26, using My Notes and OCR A Chemistry A-Level Textbook.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
How to name Amines?
Primary Amines:
When -NH2 group is on the end of the chain. Add the suffix, -amine, to the name of the alkyl chain. E.g. Ethylamine.
When -NH2 group is not on the end of the chain. Add the prefix, amino-, and a number to indicate the postion of the -NH2 group along the chain. E.g. 2-aminobutane.
Secondary or Tertiary Amines:
When the -NH, or -N is bonded to the same alkyl group, the prefixes di- and tri- are used, respectfully, the number of alkyl groups attachted to the N atom. E.g. dimethylamine and trimethylanime.
When 2 or more different groups are attachted to the N atom, the compound is named as a N-substituted dervative of the larger group. E.g. N-methlpropylamine and N-ehtyl-N-methylpropylamine.
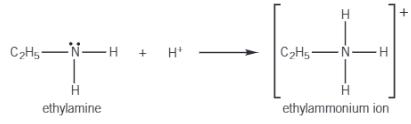
Why are Amines Bases?
Amines behave as bases, in their chemical reactions, as the lone pair of electrons on the N atom can accept a proton.
When an amine accepts a proton, a dative covalent bond is formed between the lone pair on the N atom and the proton.
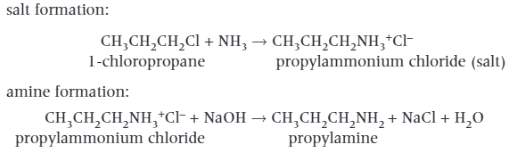
How to make Primary Aliphatic Amines?
Ammonia has a lone pair of electrons on the nitrogen atom which allows ammonia to act as a nucleophile in a substitution reaction with a haloalkane. The product of this reaction is an ammonium salt. Aqueous alkali is then added to generate the amine from the salt.
For this reaction to occur, there are some essential conditions:
Ethanol is used as a solvent. This prevents any substitution of the haloalkane by water to produce alcohols.
Excess ammonia is used. This reduces further substitution of the amine group to form secondary and tertiary animes.
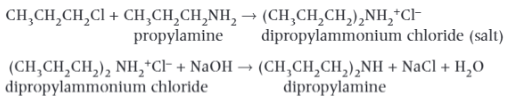
How to make Secondary and Tertiary Aliphatic Amines?
The reaction, for making Primary Amines, is unsuitable for making pure Primary Amines, as the product still contains a lone pair of electrons on the N atom that can react further with a haloalkane to form a secondary amine. The product of the 1st Reaction (In the Image) is again an ammonium salt.
The secondary amine is obtained from the salt by reacting the product with sodium hydroxide, the 2nd Reaction (In the Image).
Tertiary amines can also be formed by further reaction of the secondary amine. In this example, further substitution would form tropropylamine, (CH3CH2CH2)3N.
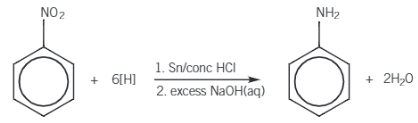
How to make an Aromatic Amine?
Phenylamine, C6H5NH2, is made by the reduction of nitrobenzene, C6H5NO2. Nitrobenzene is heated under reflux with tin and hydrochloric acid to form the ammonium salt, phenylammonium chloride, which is then reacted with excess sodium hydroxide to produce the aromatic amine, phenylamine. Tin and hydrochloric acid act as a reducing agent.
What is the Acid Hydrolysis of an Amide?
Amide + Water — H+ → Protonated Amine + Carboxylic Acid
What is the Base Hydrolysis of an Amide?
Amide + Base → Amine + Carboxylate Salt