Advanced Biochem 1: The Genetics of Diabetes
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
59 Terms
normal blood glucose levels
Fasting glucose: < 100 mg/dl
2-hour postprandial glucose (2-h PG): < 140 mg/dl
A1C: < 5.7%
pre-diabetes glucose levels
Fasting glucose: impaired, ≥ 100 - 125 mg/dl
2-hour postprandial glucose (2-h PG): impaired glucose tolerance, ≥ 140 - 199 mg/dl
A1C: 5.7% - 6.4%
diabetes glucose levels
Fasting glucose: ≥ 126 mg/dl
2-hour postprandial glucose (2-h PG): ≥ 200 mg/dl
Random PG: ≥ 200 + symptoms
A1C: ≥ 6.5%
Pathogenesis of Type 1 Diabetes Mellitus (T1DM)
Autoimmune disease (not metabolic)
• Slowly progressive disease - takes months to years
• Most common presentation is absolute insulin deficiency
→ Diabetic Ketoacidosis (DKA)
includes:
1. Autoimmune destruction of the pancreatic Beta cells
2. T cell mediated
3. Auto-antibodies bind the cellular proteins
T1DM: Autoimmune destruction of the pancreatic Beta cells
- takes months to years
- insulinitis from lymphocytic infiltration
- destruction of insulin granules
- cannot produce insulin → hyperglycemia
- 90% of β-cells are not functioning at the time of classic diagnosis
T1DM: T cell mediated
- cell invasion and apoptosis
- CD3 regulatory T cells
- CD8 natural killer T cells
- CD4 B cells
Diabetic Ketoacidosis (DKA)
body begins to break itself down to use ketones as an energy source
- because cells can no longer use the sugar in blood for energy and utilizes fats instead
- the process of burning fat produces ketones, which builds up in blood
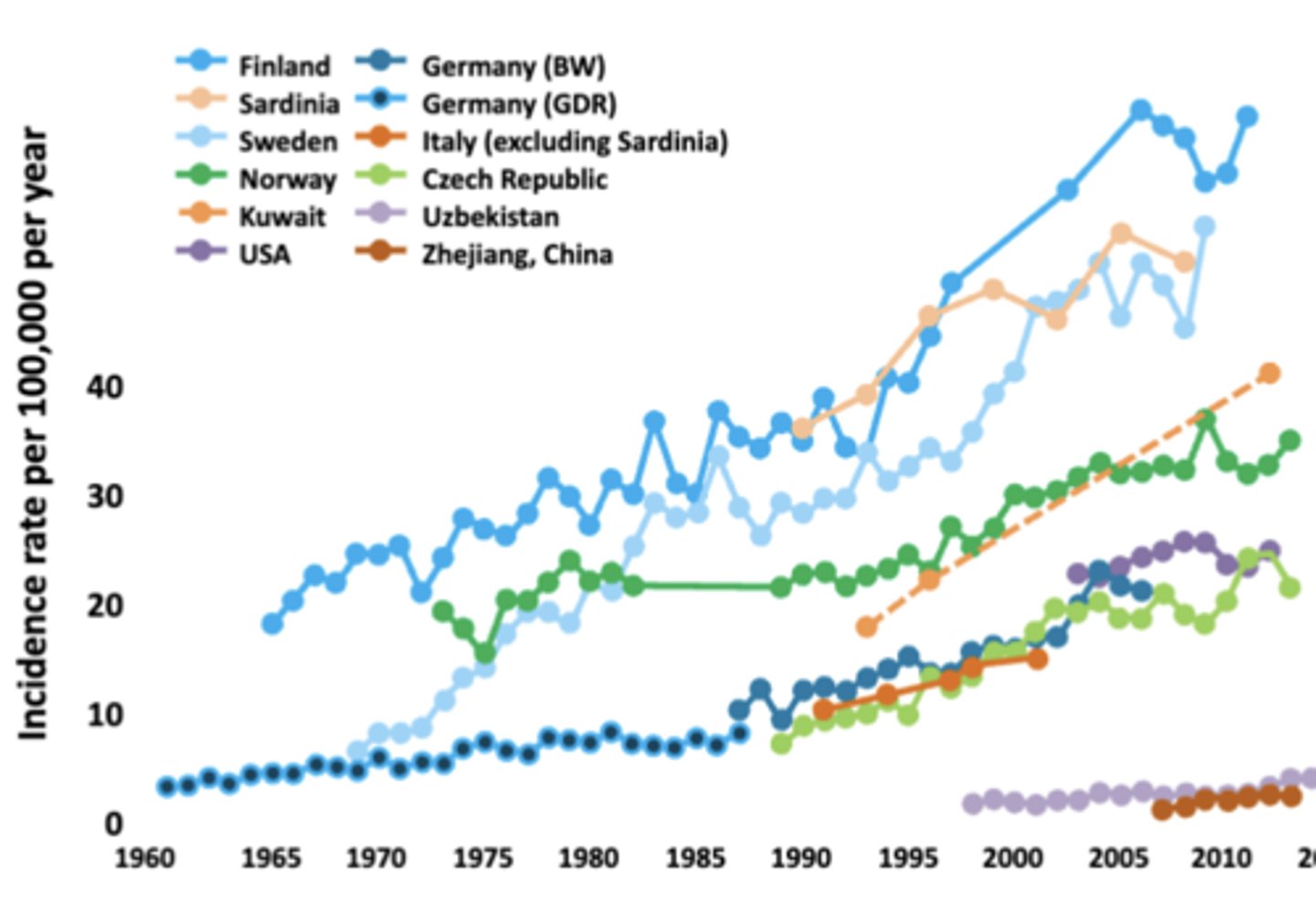
Incidence of T1D
Dramatically Increasing Worldwide
- Projected increase in T1D in US from 1.7 in 2020 to 5 million by 2050
Other Laboratory Findings for T1D
Measurable autoimmune activity
• Glutamic acid decarboxylase autoantibodies (GADA)
• Insulin autoantibodies (IAA)
• Insulinoma-associated antigen-2 autoantibodies (IA-2A)
• Zinc transporter 8 autoantibodies (ZnT8A)
Evidence of insulinopenia
• C-peptide with normal to high glucose
→ endogenous insulin
• Low or undetectable levels indicate little or no insulin secretion
Pathogenesis of T1DM-A
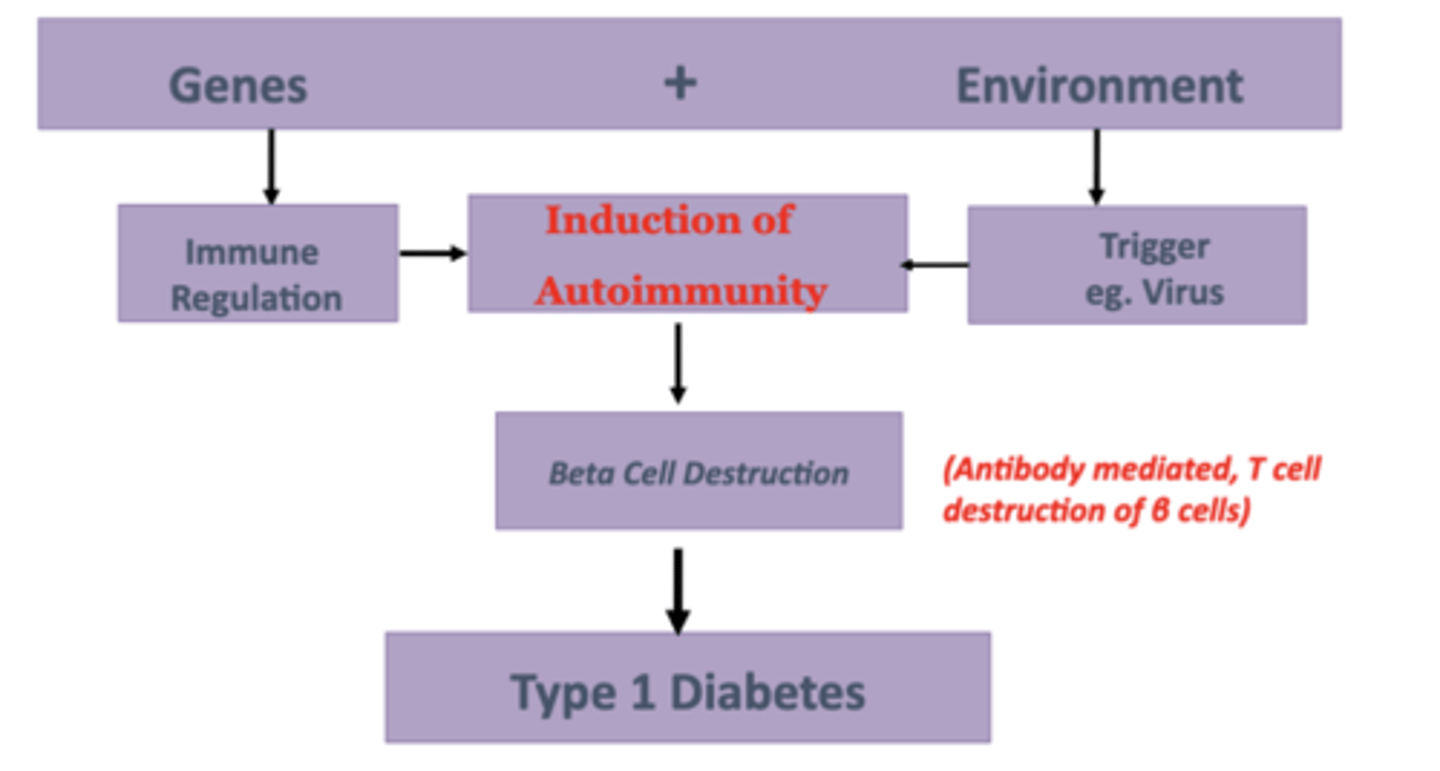
Genetics of T1DM
MHC Susceptibility Genes
- Increased Risk of T1DM
- HLA DR3 & DR4 (most closely linked)
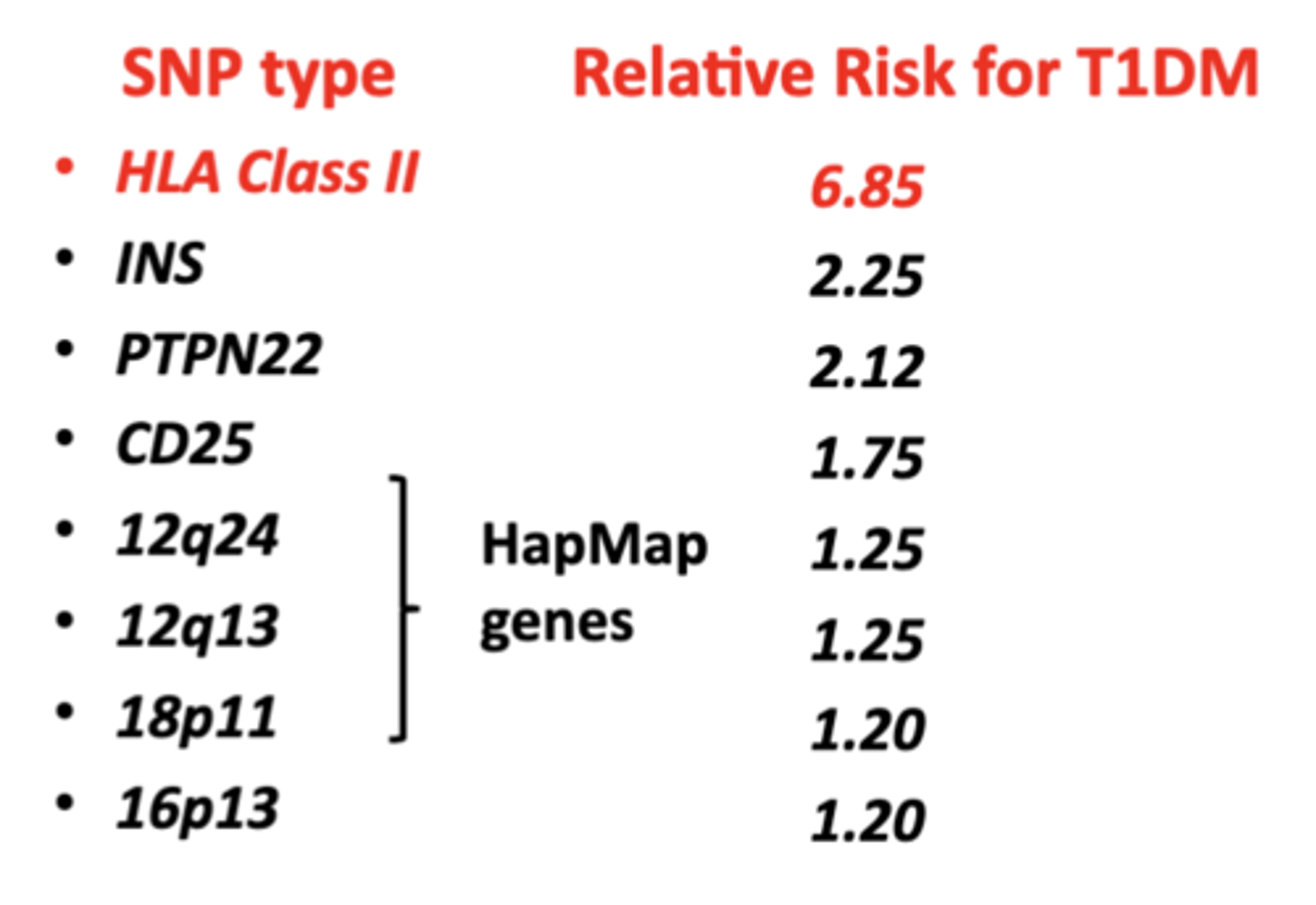
SNPs: Single Nucleotide Polymorphisms & T1DM
common type of genetic variation
- relative risk of getting T1DM can be assessed based on type of SNPs
- more susceptible to environmental triggers
- HLA Class II has the highest relative risk for autoimmunity
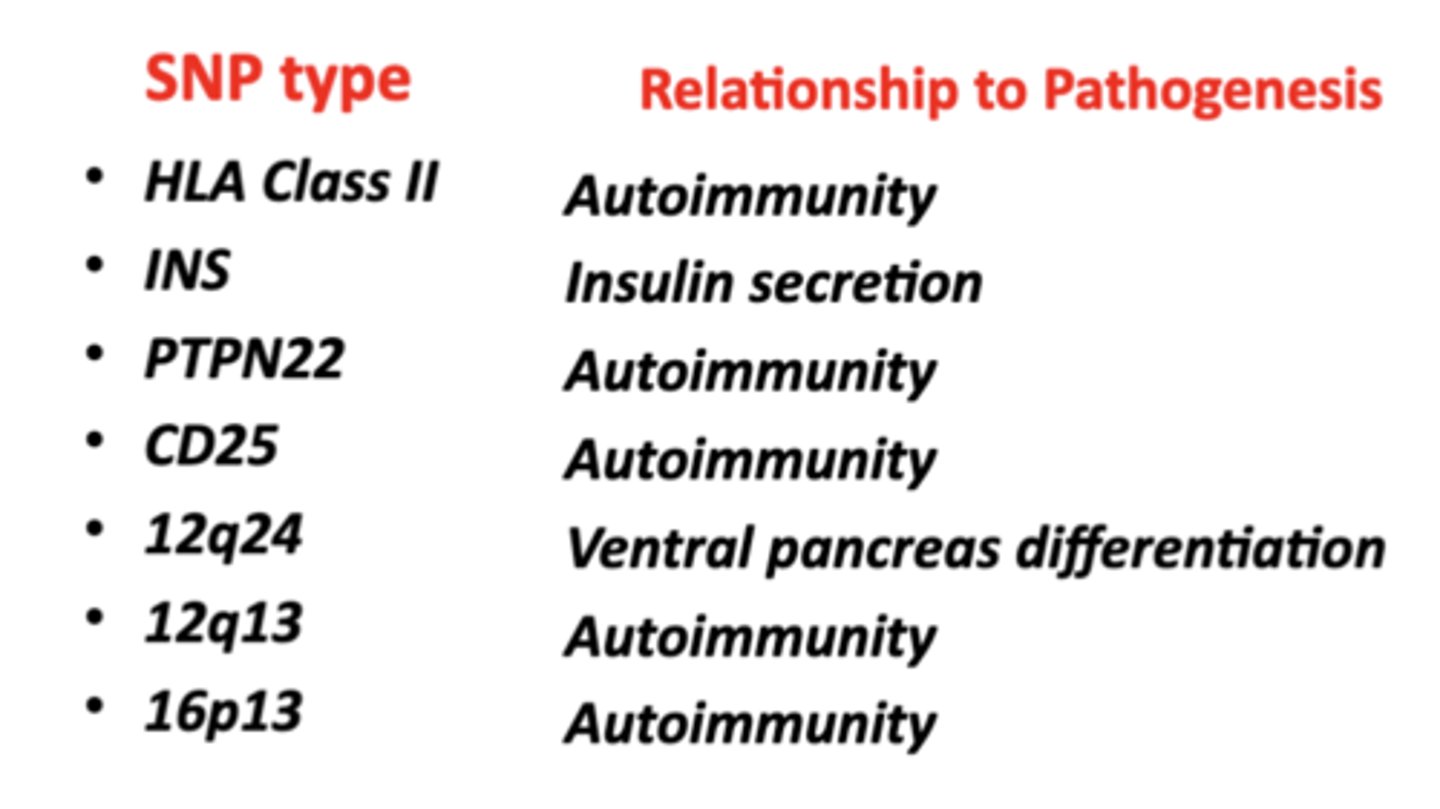
Functions of the SNP's Linked to Risk for T1DM
Familial Patterns in T1DM
• >90% of people with T1DM do not have a family history
• Spontaneous risk 0.4%
T1D Risk in Individuals Who Have an Affected Relative
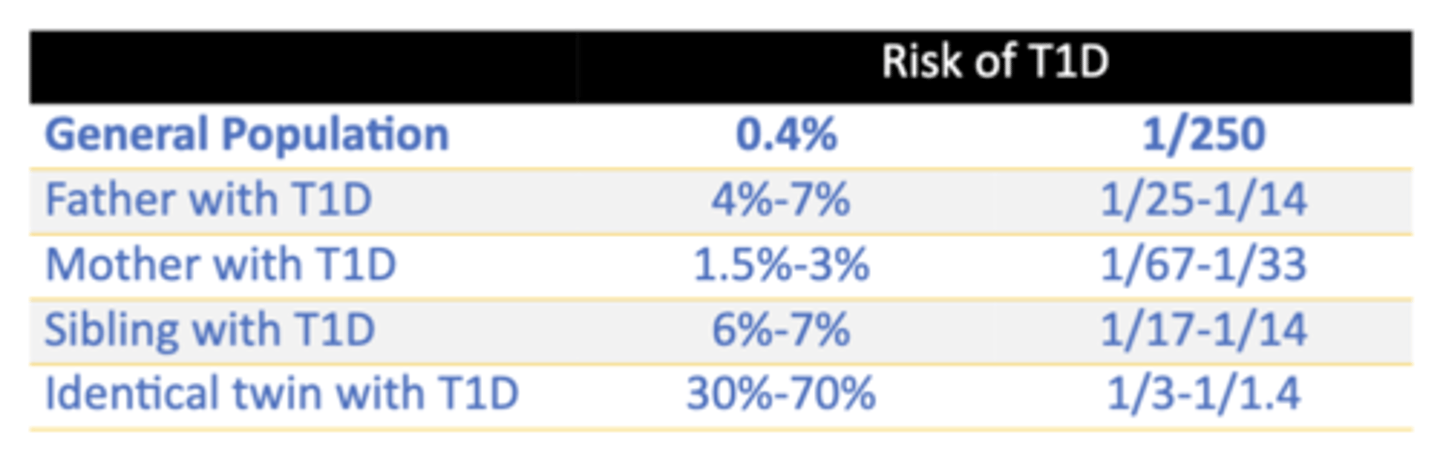
Types of T1DM
Type 1A
Type IB
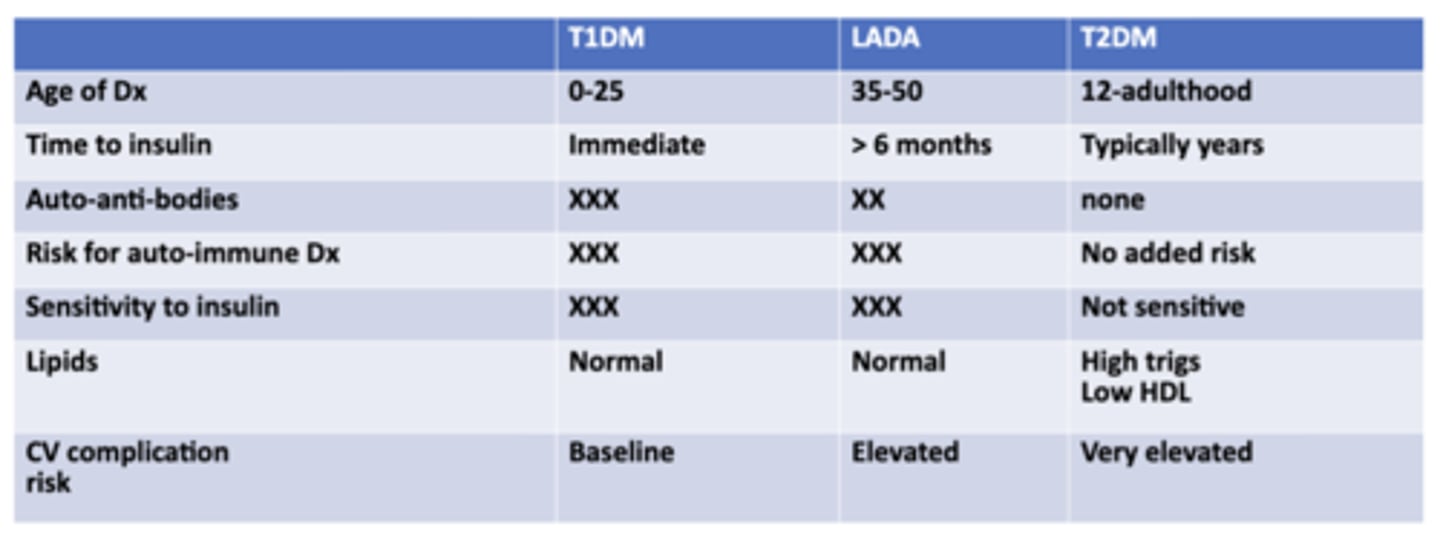
Latent Autoimmune Diabetes of Adults (LADA)
T1DM: Type 1A
presents with classic signs and symptoms of insulin deficiency
- Autoimmune response: markers of autoimmunity - GAD or ICA ATB
- Most common
T1DM: Type 1B
presents with classic signs and symptoms of insulin deficiency
- No autoimmune response no markers present
- Zinc transporter ATB are present
T1DM: LADA
slowly progressive T1DM
- Adult form of type 1
- affects people between 35-50
- Less likely to present initially with DKA
Patterns of ICA Antibody Titers Detected Over Time in the DAISY Study
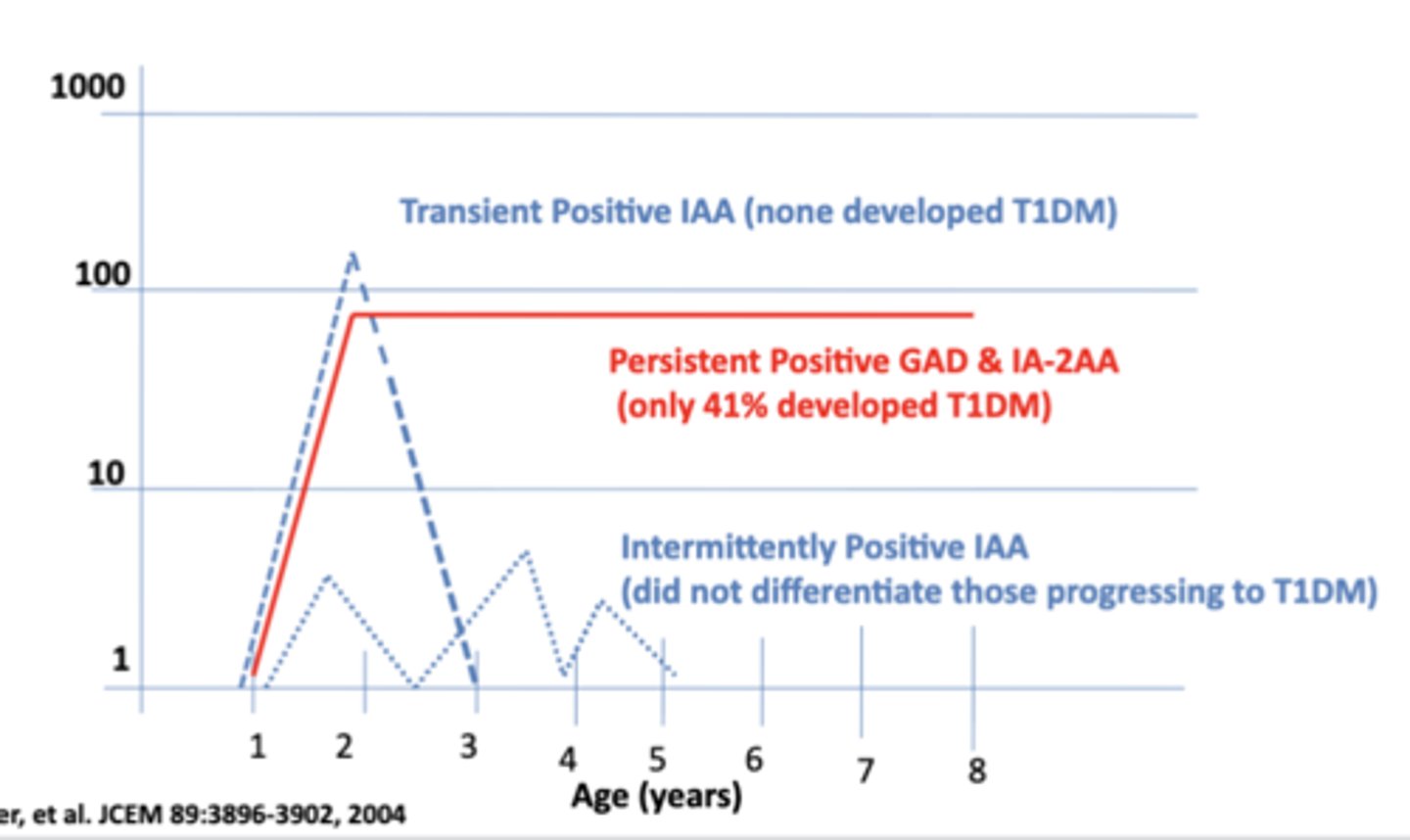
Environmental triggers of T1DM
o Viruses - most common
- enteroviruses
o Timing of immunizations
o Peri-natal factors
- lack of passing on immune protection
o Cow's milk
o Environmental toxins
o Gut microbiome
Evidence for a Viral Etiology in Pathogenesis of T1DM
• Viral-specific antibodies are present in serum in patients with new-onset T1DM
• Enteroviral RNA has been isolated from pancreatic islets of patients with new-onset T1DM
• Children with congenital rubella developed T1DM within first 5 years of life
• Cytomegalovirus (CMV) outbreaks have been associated with clusters of subsequent T1DM
• Mice inoculated with CMVB4 (obtained from a young patient with new-onset T1DM).... developed the disease
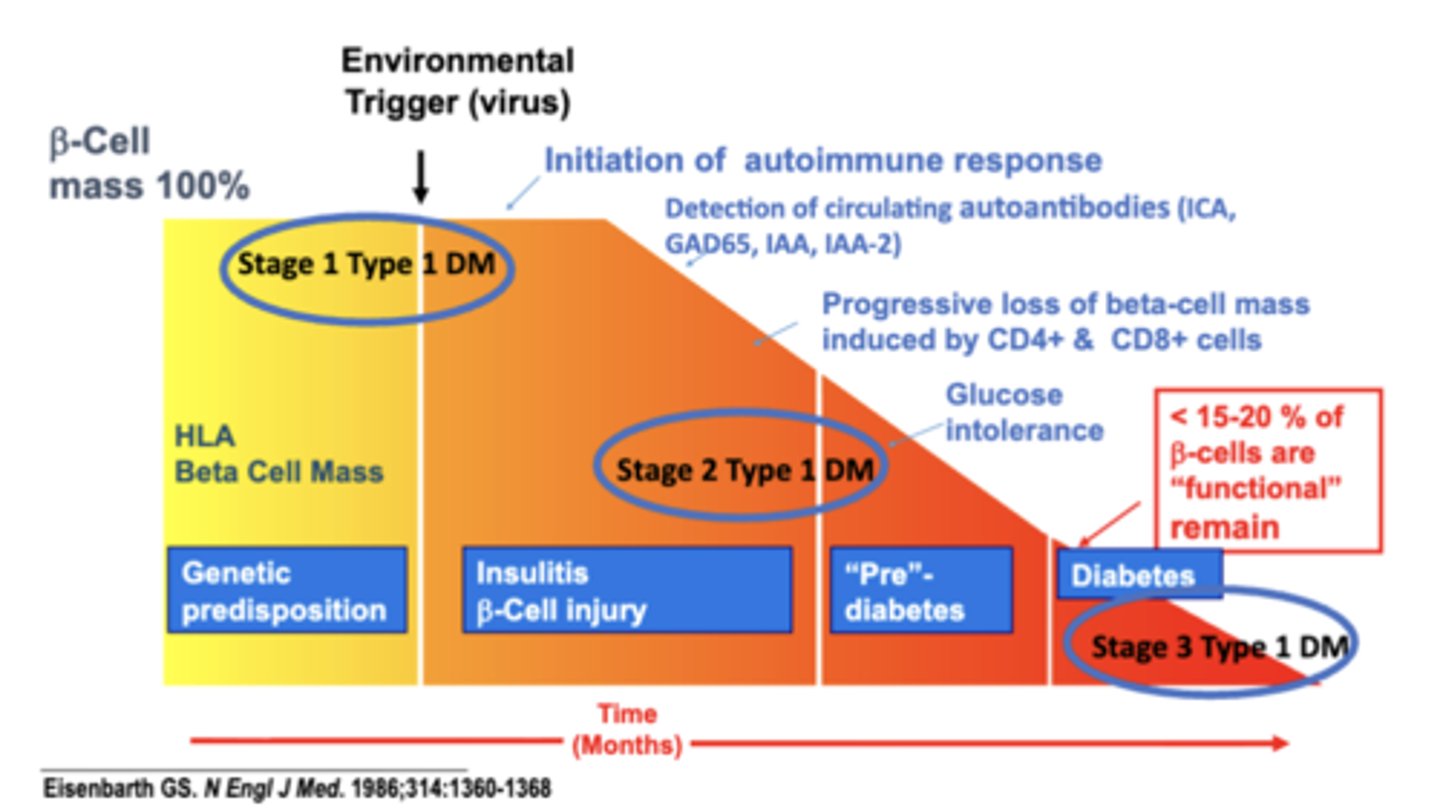
Type IA stages
Stage 1 – Initiation of autoimmune response
- Environmental Trigger (virus)
- Detection of circulating autoantibodies (ICA, GAD65, IAA, IAA-2)
- Progressive loss of beta-cell mass induced by CD4+ & CD8+ cells
Stage 2 – persistent autoimmune response
- Glucose intolerance
Stage 3 – decreased β-cells
- clinical presentation of T1DM
- < 15-20 % of β-cells are “functional” remain
- Hyperglycemia
- Symptoms
- Insulin initiation
- Diabetes by standard criteria
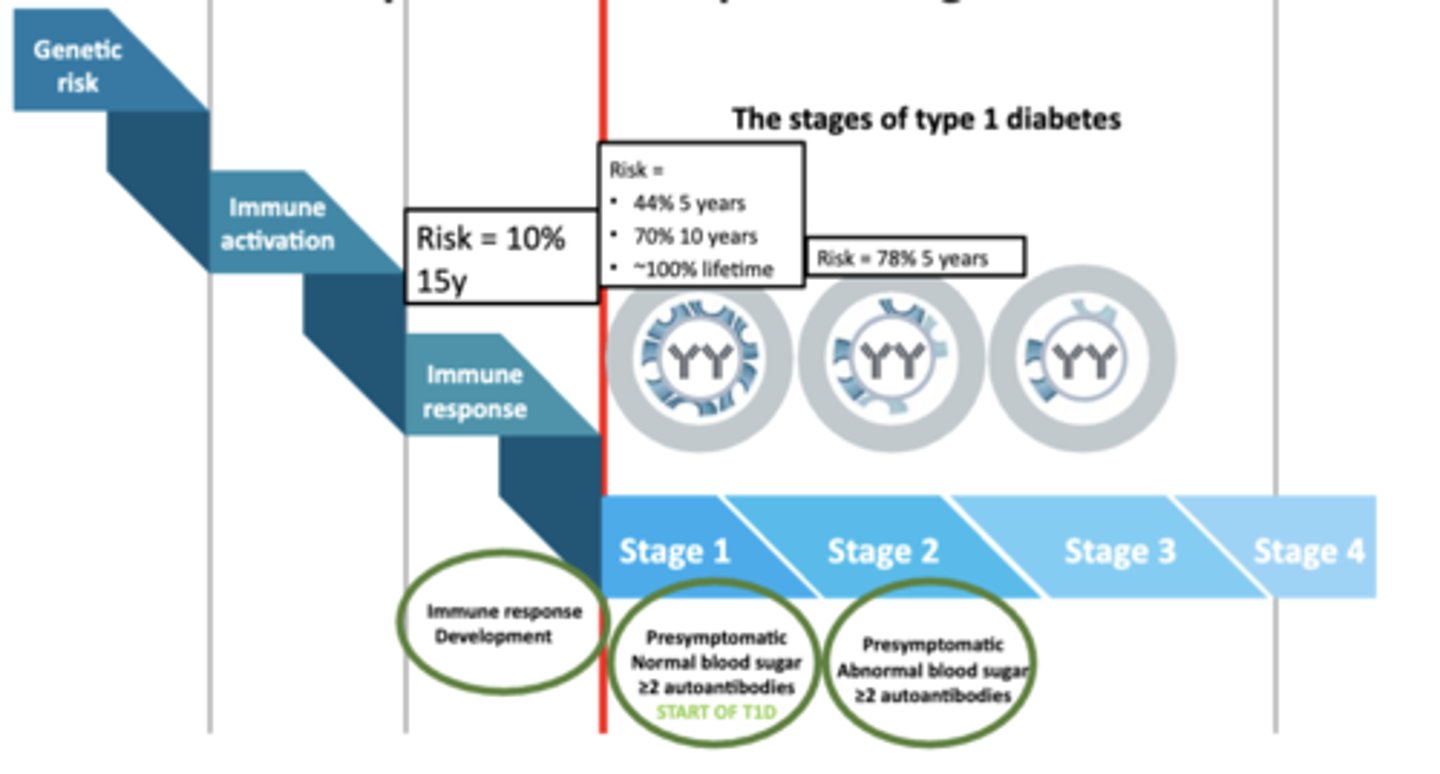
Most Prevention Trials Focus on Individuals With?
≥2 Autoantibodies Endpoint → Development Stage 3 T1D
Summary of T1DM
Autoimmune
• Need both genetic risk by HLA typing and environmental exposure
• Then sustained immune response
• We can screen by genotype and by antibody presence
• Neither is predictive enough
• We have not yet found a reasonable way to stop immune mediated Beta cell destruction
USA Regional Screening Programs
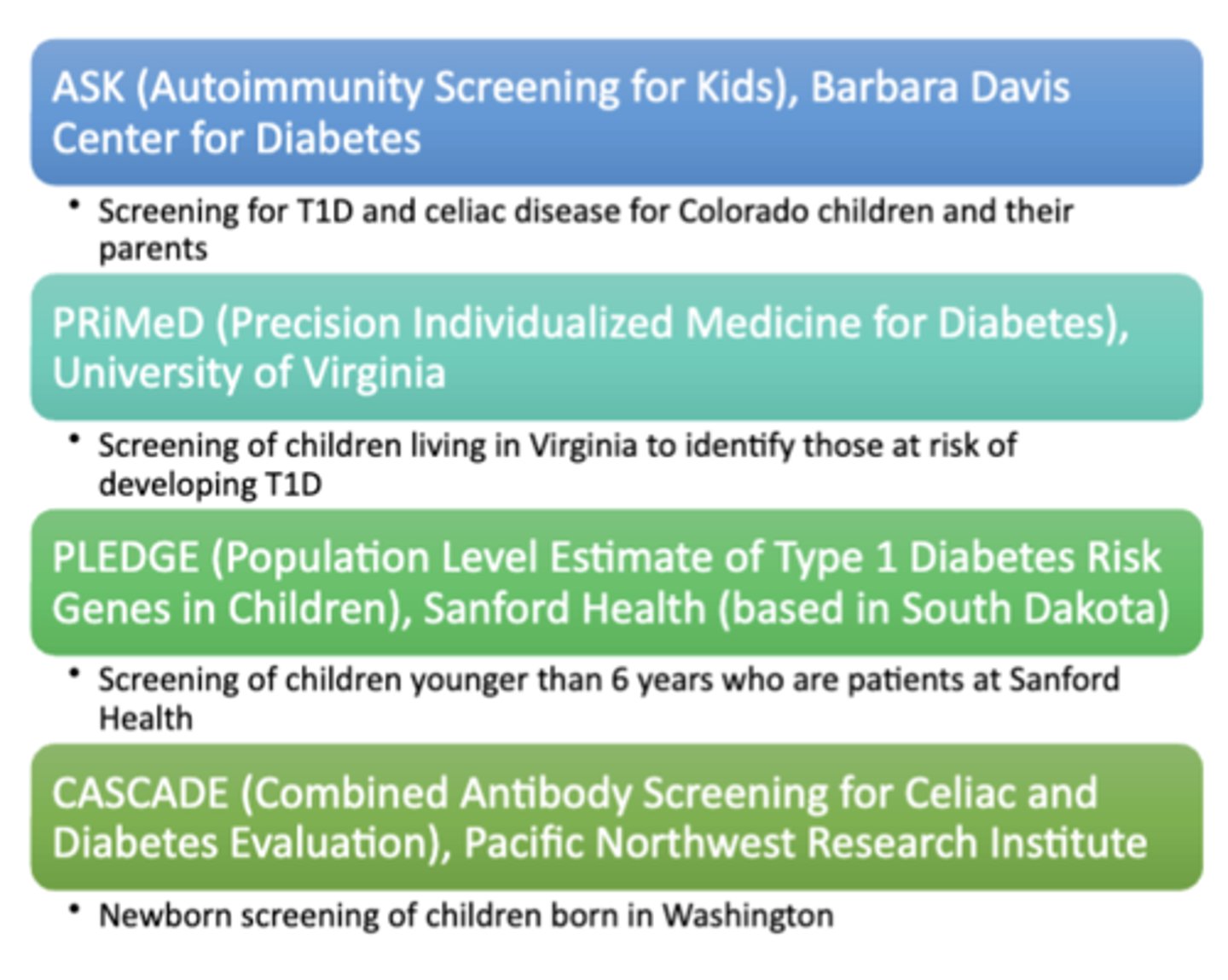
Nationally Available Screening Programs
TrialNet
- T1D research-based screening and clinical trial program for individuals who have a higher risk of developing T1D based on family history or previous autoantibody testing
T1Detect
- Initiative to increase awareness and education about screening for risk of T1Din the T1D and healthcare provider communities and to provide the general population with access to in-home T1D risk screening
Latent Autoimmune Diabetes of Adults (LADA)
− Do not show insulin resistance
− Lack of diabetes dyslipidemia
− Have carbohydrate intolerance
− Have the polys and weight loss
− Do not have a family history of LADA or insulin resistance
o Personal history of autoimmunity
o Family history of autoimmunity
When to suspect LADA
- New T1DM diagnosis age 30-50
- No FH or T2DM or insulin resistance
- Personal or FH of autoimmune disorders
- BMI < 25
- Lack of diabetes dyslipidemia
- Previous episode of the "polys" and weight loss
- Carb intolerance
Screening tool for LADA
5 Key Features:
• Age Dx less than 50
• Classic poly symptoms
• BMI < 25
• Personal history of autoimmunity
• Family history of autoimmunity
- If person have more than 2 above: 90% sensitivity, 71% specificity
- If they have 1 or zero: 99% negative predictive value against LADA
Comparing T1, T2, LADA
Diabetes in the US
1. 37.3 million Americans with diabetes (11.3%)
- 90% have type 2 diabetes
- >1-2 million more per year
2. Estimated 25% of adults > 60 y/o have DM
3. Children can have type 2
- Rates as high as 40%
- PreDM in as many as 25% of adolescence
4. 1 in 5 US health care dollars spent on person with DM
PRE-Diabetes in the US
1. 100 million Americans have pre-DM (37%)
• 85% do not know they have it
2. CA– 55% of adults have DM or pre-DM
3. CDC predicts people born in 2000
• 1 in 3 will develop DM
• 40% risk in at risk populations
• 50% or 1 in 2 in Hispanic population
Type 2 Diabetes Mellitus (T2DM)
− Metabolic disease characterized by insulin resistance
− Individuals secrete insulin but cannot use it by cells → this is termed insulin resistance
Insulin resistance can be induced by?
a number of factors:
- increased weight
- increase caloric intake
- increased sedentary lifestyle
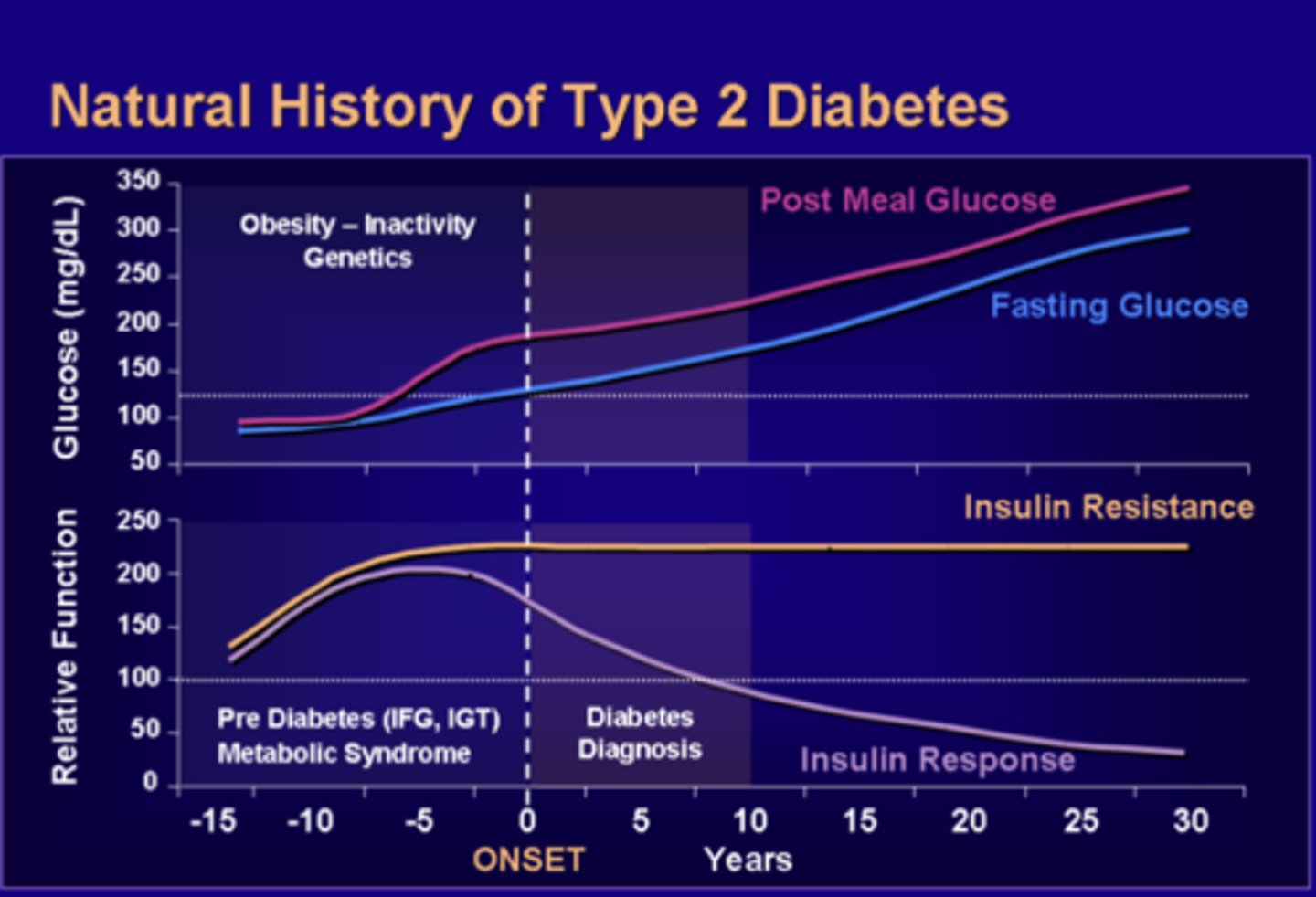
Natural History of Type 2 Diabetes
1. The pancreas compensates by producing more and more insulin in order to bring glucose into the cells
- however, sugar continues to build up in the blood
2. T2DM takes a long time to develop
- most of the time patient is unaware due to it being asymptomatic
3. Increased in insulin resistance leads to compensatory increases in circulating insulin
- prevents glucose levels from being too high
4. As time goes on, insulin resistance reaches a peak and plateaus
- compensatory release of insulin continues to prevent fasting glucose levels from being too high
5. β-cells reaches capacity and as the fasting glucose level remain normal, post-meal glucose level rises
- Insulin cannot keep up with β-cell dysfunction and both fasting and post-meal glucose levels rose over time.
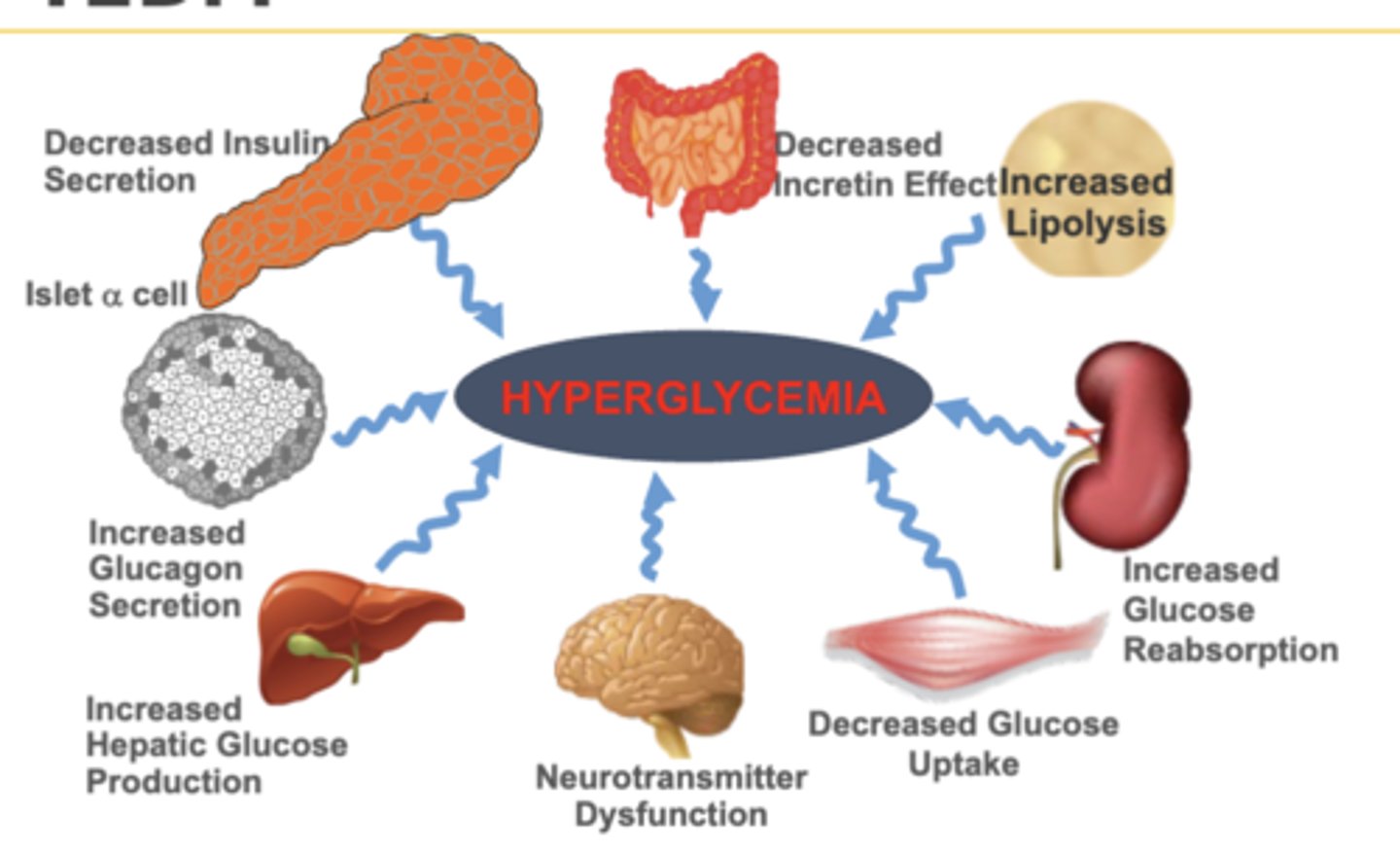
Multiple metabolic defects for T2DM
1. Decreased insulin secretion
o Decreased sensitivity and years of resistance
2. Increased glucagon secretion
o Increased gluconeogenesis → hyperglycemia
3. Increased hepatic glucose production
o Liver makes glucose at night when fasting die to inability to detect glucose
4. Neurotransmitter dysfunction
o Either never satisfied or always satiated
5. Decrease glucose uptake
o Increase glucose in blood → hyperglycemia
6. Increase glucose reabsorption
7. Increase lipolysis
8. Decreased incretin effects
Type 2 Diabetes Genetics and Inheritance
1. Medically complex disorder
• >100 genetic alleles tied to T2DM
• 8+ Pathophysiologic mechanisms
2. Family History is a strong RF
• Risk T2DM is
• 1 in 7 if a parent dx before 50 years
• 1 in 13 if parent dx after age 50
• 1 in 2 if both parents have T2DM
3. Most people likely have pre-existing pre-diabetes
• Approximately 11%/ year progress
• 70% will progress over a lifetime
Who Should Be Tested for T2DM?
Any patient with BMI ≥ 25 and a risk factor:
• Physical inactivity
• First-degree relative with DM
• High-risk race/ethnicity: African American, Latino, Native American, Asian American, Pacific Islander
• History of Gestational Diabetes
• Hypertension
• HDL < 35 or Triglycerides > 250
• History of Polycystic Ovarian Syndrome (PCOS)
• HgbA1c ≥ 5.7 or fasting glucose > 100
• Acanthosis nigricans
• History of CVD
Who Should Be Tested for T2DM? No Risk Factors + Normal BMI
• Begin testing at age 35
• Repeat testing every 3 years if normal
• Annually if pre-diabetes
US Preventive Services Task Force:
• Screen asymptomatic patient for DM if BP > 135/80
Diabetes Prevention Program
1. NIH Diabetes Prevention Program (DPP) Study
2. Nationwide clinical study
• 3000 adults at high risk for developing T2DM across 27 different centers
3. Intervention methods
• Routine care
• Metformin (titrated gradually)
• Lifestyle intervention:
- Reduction in fat & calorie intake
- Physical activity of 150 minutes per week (minimum)
- Weight loss of ≥ 7% body weight and maintained of weight loss
Interventions and Reduced Risk of T2DM
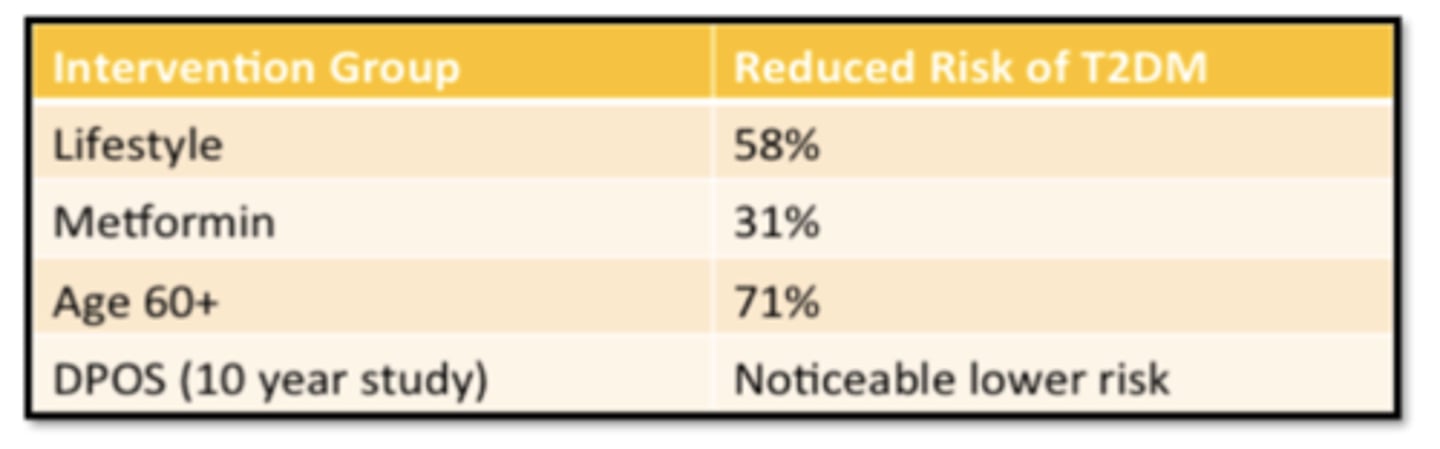
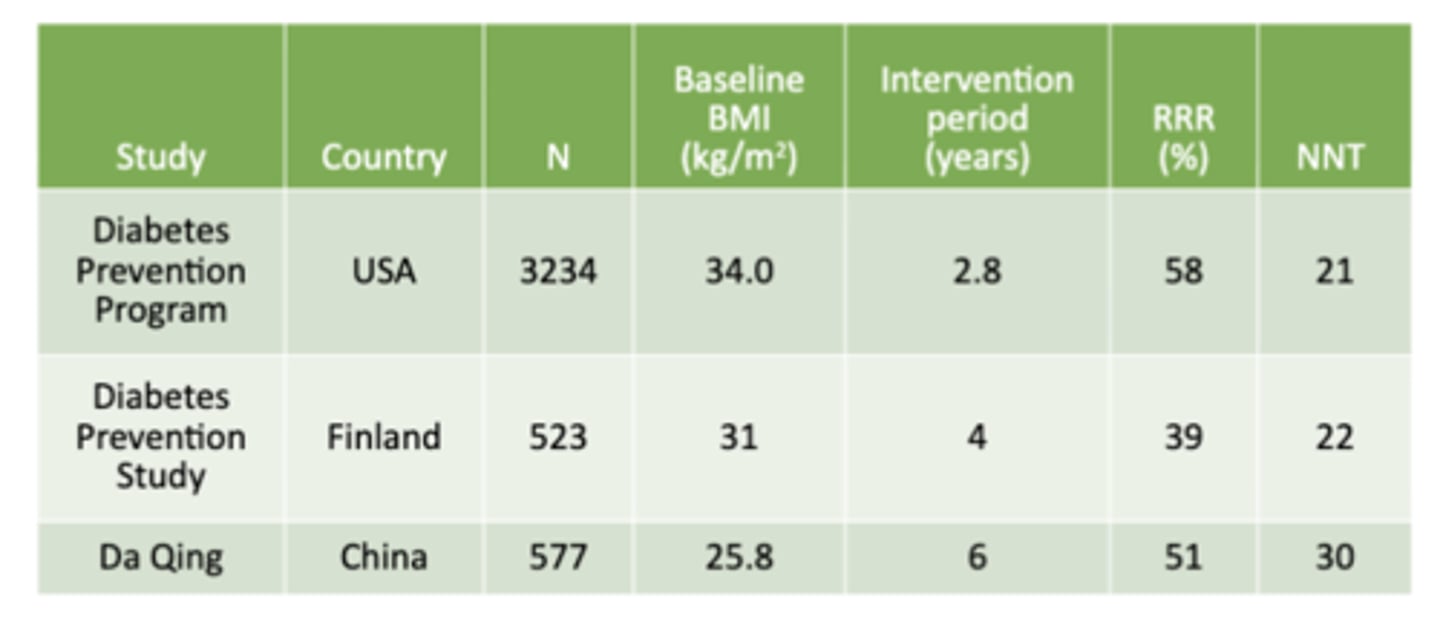
Prevention of T2D: Selected Lifestyle Modification Trials
T2D Clinical Presentation
Most often asymptomatic
• Found at routine testing
• Found at time of diagnosis of complication
• Found at other medical care
T2D Symptoms
not specific
• Tiredness
• Blurred vision
• Headaches
- "polys" and weight loss represent decompensation from long term abnormalities
Monogenic diabetes of young (MODY)
characterized by:
- non-insulin dependent diabetes diagnosed at a young age (<25 yrs)
- lack of autoantibodies
- Phenotypically, people with MODY appear like T1DM patients
- Clinically heterogeneous disorder
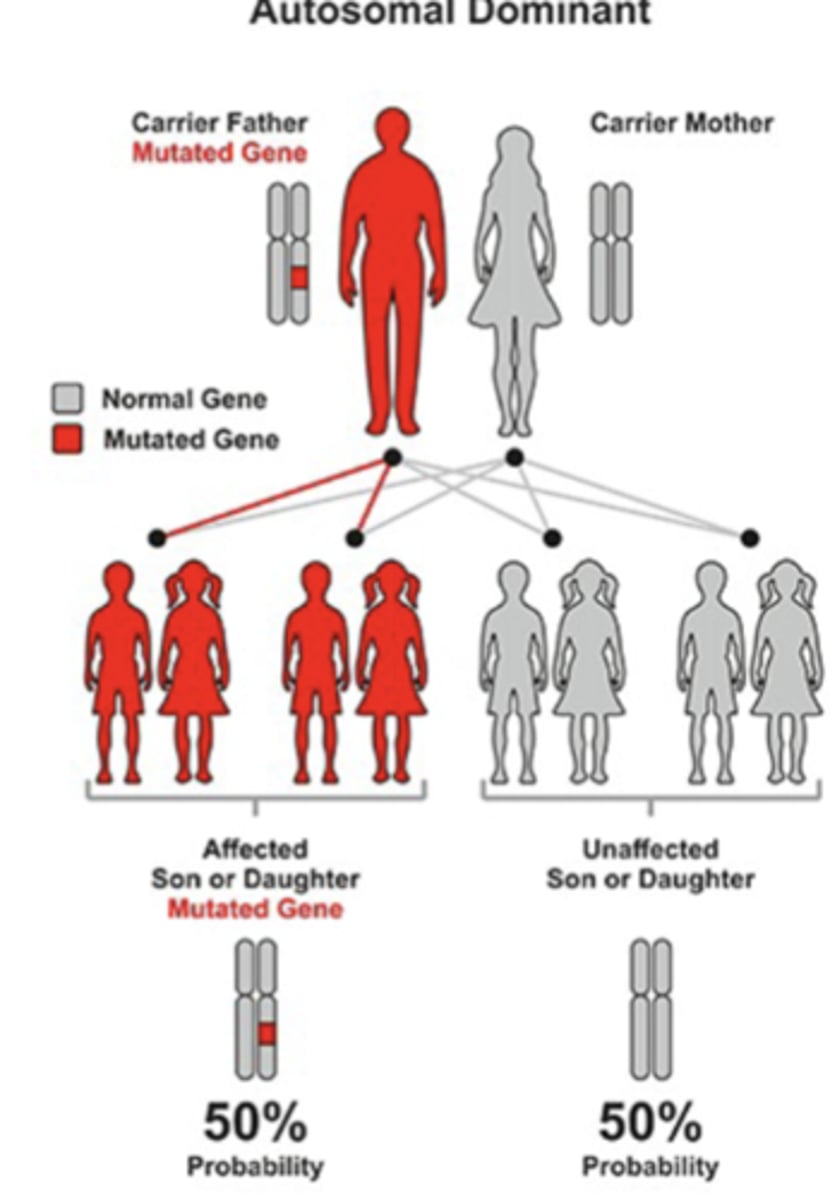
MODY Inheritance
Clinically heterogeneous disorder:
- autosomal dominant transmission
- Not metabolic abnormality or autoimmune
- Very strong family history is expected
- Caused by a single genetic abnormality:
o Impaired glucose sensing
o Impaired insulin secretion with minimal or defect in insulin action
o defect in the way insulin is packaged, made, or secreted
MODY Treatment
includes a drug that forces insulin to be secreted more quickly
- they do not need to be given insulin
MODY - form of monogenic diabetes
1. 500,000 in US (compared to 2 million T1DM)
2. Autosomal Dominant
- Each child of a parent with MODY has 50% chance of inheriting the disease
3. Collection of β-cell defects from gene mutations
- Genes control β-cell development, function and regulation
- Mutation in genes → impaired glucose sensing, insulin secretion with minimal or defect in insulin action
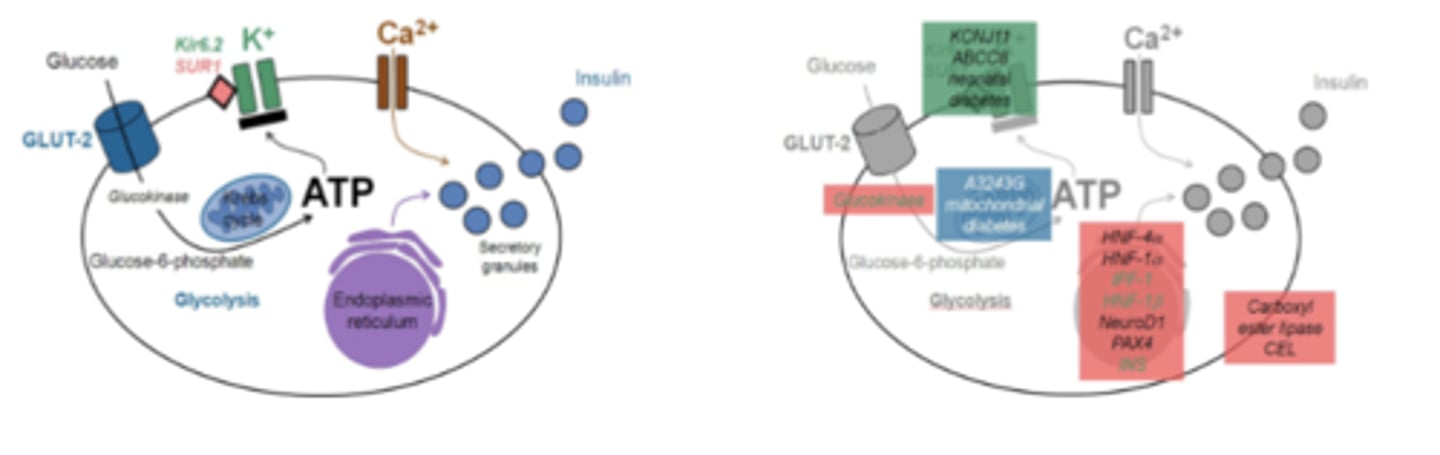
Monogenic Diabetes Pathogenesis
Pancreatic beta cell: coupling of:
- glucose sensing via GLUT-2 transporters
- generation of ATP
- membrane depolarization by closing potassium channels
- entering of calcium ions
- exocytosis of insulin
Location in the pancreatic beta cell of the:
1. gene mutations
2. affected proteins
- MODY(red)
- mitochondrial diabetes (blue)
- neonatal diabetes (green)
MODY Diagnosis
Diagnosis most often made by history
• Age is less predictive
• Can present early in life
• Can be missed till middle age
Genetic testing possible
• Clinical testing
• Research testing
MODY 3
Most common form 50-60%
• HNF Alpha defect
• Post prandial hyperglycemia
Treatment: low dose sulfonylurea
MODY 2
15 – 31%
• Glucokinase defect
• Mild, stable fasting hyperglycemia
• Not progressive, often diagnosed on routine screening
Treatment: diet (low carb)
MODY 1
Less common 10%
• Defective gene HNF-4
• High post prandial blood glucose is most commonly seen
Treatment: typically responds to SU
MODY 5
• Early diabetes +/- renal disease (cysts)
• HNF-1-β defect
MODY 8
Exocrine pancreatic insufficiency
Other Types of MODY
- MODY 4 (IPF 1)
- MODY 6 (NERUOD1)
- MODY 7 (KLF 11)
- MODY9 (PAX4)
- MODY 11(BLK) - very rare
Comparing Types of Diabetes
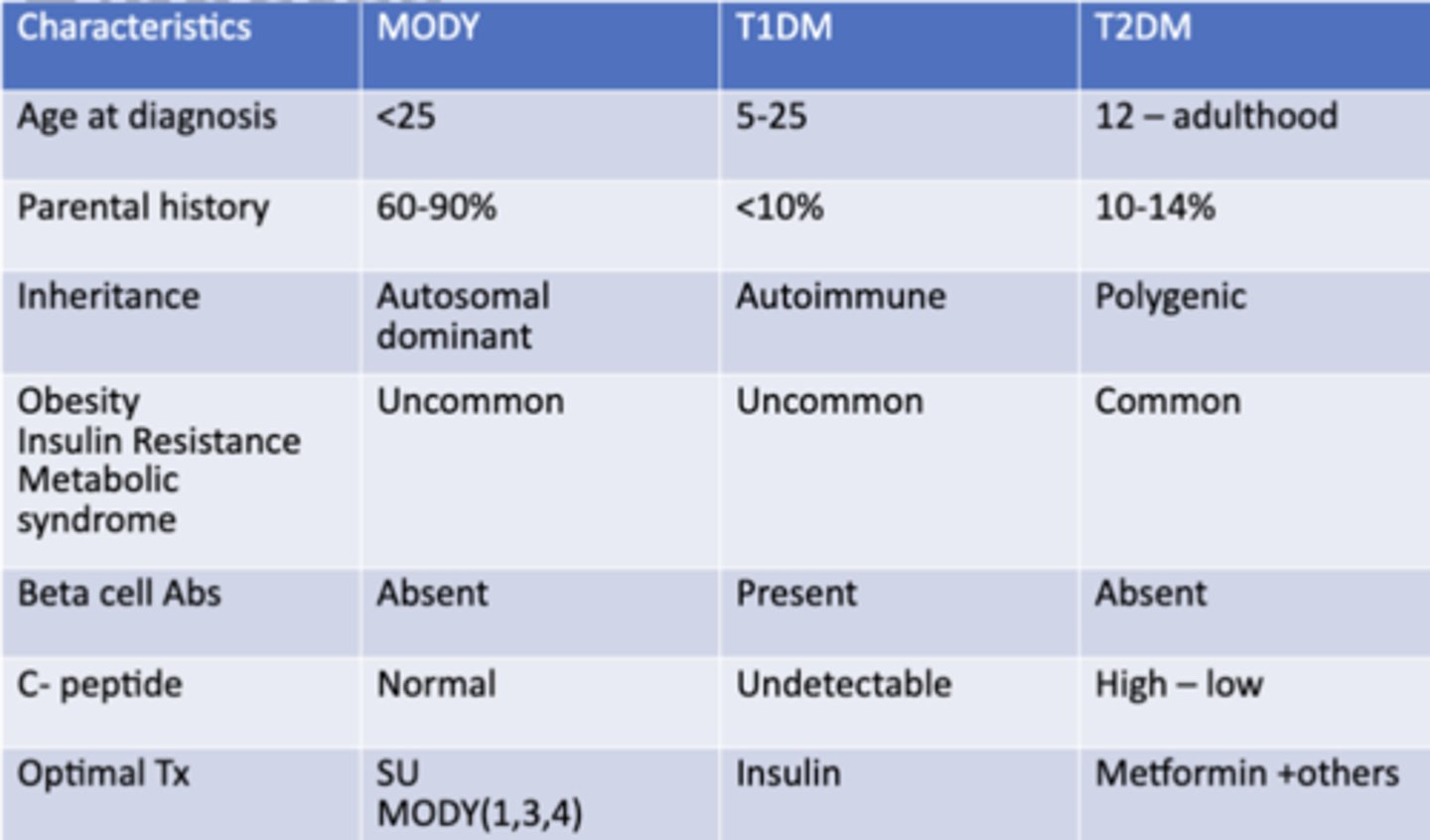
Other diabetes mellitus types
• Ketosis prone diabetes
• Secondary Diabetes
- (type 3c or pancreatogenic)
- Steroid induced
• Type 5 Diabetes mellitus