Representing Molecules
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
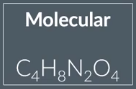
Molecular formula
actual number of atoms of each element in a molecule
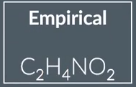
Empirical formula
simplest whole-number ratio of elements in a compound.
How to find molecular formula
counting the number of atoms of each element
listing the carbon and hydrogen atoms first
listing non-carbon or hydrogen elements in alphabetical order
How to find empirical formula
find simplest whole nuber ratio of the elemnents ( divide by a common factor so long as each atoms proportion is a whole number)
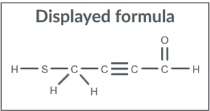
Displayed Formula
position of every atom and every bond
single bonds as one line ( C—C)
double bonds as two lines ( C═O)
triple bond as three lines ( C≡N)
How to draw displayed formula
draw the non-hydrogen atoms eg) C, N, Br, O
fill in the hydrogens with the correct carbon bonds
you must show every bond and atom to get the mark
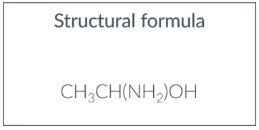
Structural formula
position of all atoms in a molecule without showing any of the individual bonds
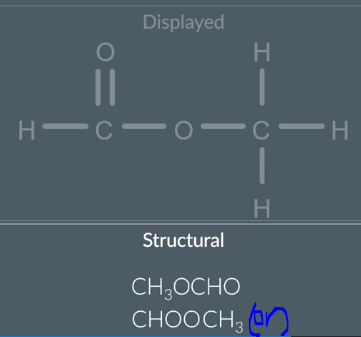
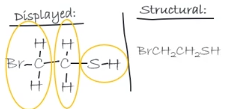
Displayed→ Structural
Collapse any non hydrogen atom along with any other non hydrogen atom into one group
then remove the bonds
reorganise the symbols of each group in alphabetical eg) C 1rst , H 2nd , N 3rd, O 4th
put altogether
when you have a regular CH3 group, we write that first/as the first cabon
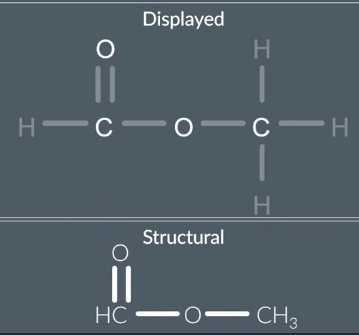
Molecular → Structural Formula
draw the displayed then group atoms together
the draw structural
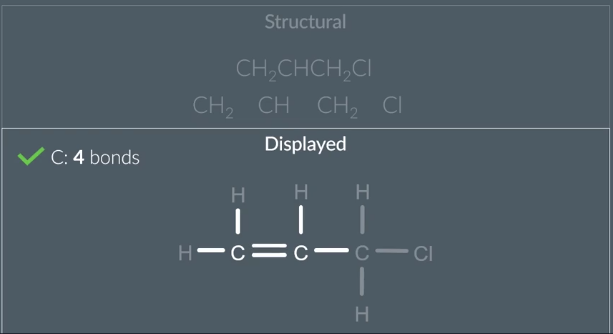
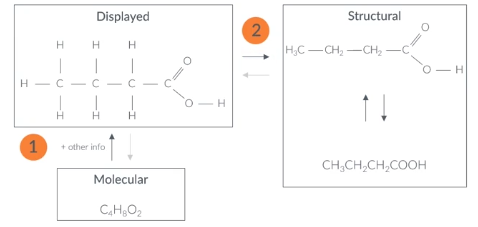
Structural→ Displayed
locate the groups withing the molecule of structural formula
then draw all non hydrogen bonds with single lines
fill in the hydrogens
check to see if relavent atoms have the number of bonds theyre meant to eg ) C=4 bonds O=2 bonds
if carbon doesnt have 4 bonds it means it needs a double/triple bond
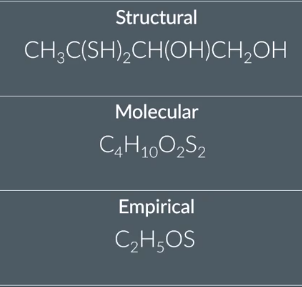
Structural → Molecular →Empirical
count up the no of atoms of each element first
list the carbon and hydrogen atoms first in alphabetical order
then use the molecular formula to deduce the simplest whole number ratio of atoms