PHPOrgChem (Lecture) | Module 7: (Part 1: ALCOHOLS, PHENOLS, and THIOLS ONLY)
1/133
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
134 Terms
Alcohol
Contain the hydroxyl (-OH) group.
Ethanol
ALCOHOLS: found in antiseptics and alcoholic beverages
Primary Alcohol, Secondary Alcohol, and Tertiary Alcohol
Alcohols are classified as?
Primary Alcohol
ALCOHOLS CLASSIFICATION: -OH attached to a carbon with one other carbon.
Primary Alcohol
ALCOHOLS CLASSIFICATION: Ex.: Ethanol & Methanol
Primary Alcohol
ALCOHOLS CLASSIFICATION: Attached to one alkyl group and/or hydrogen
Primary Alcohol
ALCOHOLS CLASSIFICATION: More reactive
Primary Alcohol
ALCOHOLS CLASSIFICATION:
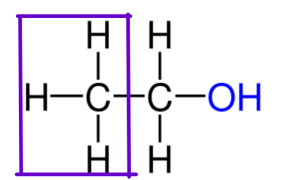
Primary Alcohol
ALCOHOLS CLASSIFICATION:
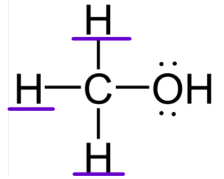
Secondary Alcohol
ALCOHOLS CLASSIFICATION: -OH attached to a carbon with two other carbons.
Secondary Alcohol
ALCOHOLS CLASSIFICATION: Ex. 2-propanol
Secondary Alcohol
ALCOHOLS CLASSIFICATION:
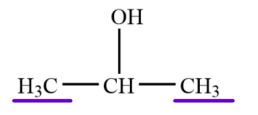
Tertiary Alcohol
ALCOHOLS CLASSIFICATION: -OH attached to a carbon with three other carbons.
Tertiary Alcohol
ALCOHOLS CLASSIFICATION: Ex.: 2-methyl-2-propanol
Tertiary Alcohol
ALCOHOLS CLASSIFICATION:
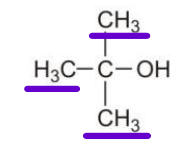
Polar
PHYSICAL PROPERTIES: Alcohols are ____ due to the hydroxyl (-OH) group.
Water
PHYSICAL PROPERTIES: Alcohol are polar, ethanol can easily dissolve in ____
Shorter
PHYSICAL PROPERTIES: Alcohols can mix well with water esp. those with ______ carbon chain
Inversely Proportional
PHYSICAL PROPERTIES: Solubility of Alcohol decreases as carbon chain length increases (____________)
Hydrogen
PHYSICAL PROPERTIES: Alcohols can form ________ bonds
Alkanes
PHYSICAL PROPERTIES: Alcohols have higher boiling points than ______, due to strong Hydrogen bonding
More Heat
PHYSICAL PROPERTIES: Alcohol and water require ________ to evaporate
Less
PHYSICAL PROPERTIES: Alcohol is _____ dense than water
Colorless
PHYSICAL PROPERTIES: What is the color of the liquid (Alcohol)?
Sweet
PHYSICAL PROPERTIES: Alcohols have mild, sometimes ____ smell
Weak
PHYSICAL PROPERTIES: Alcohols are _____ acid → can donate Hydrogen ion but not as easily like acetic acid
Acetic Acid
PHYSICAL PROPERTIES: Alcohols are weak acid → can donate Hydrogen ion but not as easily like?
Oxidation
REACTIONS WHERE ALCOHOLS UNDERGO: one of their most useful properties
ALDEHYDES, KETONES, or CARBOXYLIC ACID
REACTIONS WHERE ALCOHOLS UNDERGO: Depending to the structure of the alcohol, oxidation can produce?
Primary Alcohol
OXIDATION OF ALCOHOLS: ________ → Aldehydes → Carboxylic acids.
Second Alcohol
OXIDATION OF ALCOHOLS: _______ → Ketones.
Tertiary Alcohol
OXIDATION OF ALCOHOLS: ________ → resist oxidation.
Ethanol
REACTIONS WHERE ALCOHOLS UNDERGO: Oxidation of _____ in the liver → produces acetaldehyde → responsible for hangover symptoms
Acetaldehyde
REACTIONS WHERE ALCOHOLS UNDERGO: Oxidation of ethanol in the liver → produces ________ → responsible for hangover symptoms
Combustion
REACTIONS WHERE ALCOHOLS UNDERGO: Can BURN IN OXYGEN producing CARBON DIOXIDE AND WATER in a process called?
Carbon Dioxide, and Water
REACTIONS WHERE ALCOHOLS UNDERGO: Alcohols can burn in oxygen producing?
Ethanol
COMBUSTION OF ALCOHOLS: This undergo combustion → product is used in biofuels
Dehydrated
REACTIONS WHERE ALCOHOLS UNDERGO: Can be ______ (LOSE WATER) to form ALKENES when treated with strong acids
Carboxylic Acid
REACTIONS WHERE ALCOHOLS UNDERGO: Can react with ___________ TO FORM ESTERS in a process called ESTERIFICATION
Esters
REACTIONS WHERE ALCOHOLS UNDERGO: Can react with Carboxylic Acid to form this
Propranolol
1-NAPHTHALEN-1-YLOXY-3-(PROPAN-2-YLAMINO)PROPAN-2-OL is also known as?
Propranolol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: ANTIHYPERTENSIVE MEDICATION (Beta-Blocker)
Secondary Alcohol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: What is the classification of the alcohol in Propranolol?
Water
EXAMPLES OF DRUGS CONTAINING ALCOHOLS:
SOLUBILITY: The alcohol group makes propranolol dissolves well in ____, helping it to be absorbed into the bloodstream and reach its target site
Liver
EXAMPLES OF DRUGS CONTAINING ALCOHOLS:
METABOLISM: drug metabolize in the _____ where the alcohol group influence how long the drug last in the body, and how quickly it is broken down
Extreme Conditions
EXAMPLES OF DRUGS CONTAINING ALCOHOLS:
STABILITY: The alcohol group helps maintain propranolol stability when stored properly, but ___________ can affect effectiveness (air exposure → when the drug is opened and exposed to air/moisture)
Blood-brain barrier
EXAMPLES OF DRUGS CONTAINING ALCOHOLS:
EFFECTIVENESS:
Alcohol group allows propranolol to cross the?
Propranolol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: Use to treat anxiety & heart conditions by acting on the heart and brain
Stage Fright
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: Before, propranolol is use only for heart conditions, but now, propranolol can be used for?
Albuterol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: Name of SALBUTAMOL used in US/western countries
Salbutamol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: “ALBUTEROL” (name used in US/western countries)
Salbutamol
EXAMPLES OF DRUGS CONTAINING ALCOHOLS: Alcohol helps it dissolve in water and be effective in treating asthma and other respiratory conditions by improving absorption and delivery into the lungs.
Bronchodilator
EXAMPLES OF DRUGS CONTAINING ALCOHOLS:
EFFECTIVENESS: Alcohol group aids in the drugs’ ability to reach its target in the lungs
Where it works as _________ to relax the muscle in the airways helping to ease breathing
Phenol
A TYPE OF ARENE WITH ALCOHOL GROUP
Phenol
Differs from alcohol because of their hydroxyl group (-OH) is attached to an aromatic ring (arene) which makes them more stable, and more acidic compared to alcohol
More Stable, and More Acidic
PHENOL: Differs from alcohol because of their hydroxyl group (-OH) is attached to an aromatic ring (arene) which makes them __________, and __________ compared to alcohol
Arene
a cyclic ring with six carbons and alternating double bonds, which make them more stable and more acidic.
Phenol
More acidic than alcohols due to resonance stabilization.
Phenol
Used in disinfectants and pain relievers
Phenol
are special type of organic compounds where the hydroxyl group is directly attached to a benzene ring
Polar
PHYSICAL PROPERTIES: ____- due to the hydroxyl (-OH) group attached to an aromatic benzene ring.
Phenol
PHYSICAL PROPERTIES: They mix somewhat with water but their SOLUBILITY IS LOWER compared to small alcohols like ethanol, however they DISSOLVE WELL IN ORGANIC SOLVENTS
Lower
PHYSICAL PROPERTIES: Phenols mix somewhat with water but their SOLUBILITY IS ______compared to small alcohols like ethanol, however they DISSOLVE WELL IN ORGANIC SOLVENTS
Organic Solvents
PHYSICAL PROPERTIES: Phenols mix somewhat with water but their SOLUBILITY IS LOWER compared to small alcohols like ethanol, however they DISSOLVE WELL IN?
Phenol
PHYSICAL PROPERTIES: Unlike regular alcohols, they have HIGHER BOILING POINTS because they form STRONG HYDROGEN BOND, making them HARDER TO EVAPORATE
Strong Hydrogen Bond
PHYSICAL PROPERTIES: Unlike regular alcohols, Phenols have HIGHER BOILING POINTS because they form ___________________, making them HARDER TO EVAPORATE
Moderately Soluble
PHYSICAL PROPERTIES: Phenols are _____________ in water but MORE SOLUBLE in organic solvents.
Non-Polar
PHYSICAL PROPERTIES: Solubility of Phenols decreases as the benzene ring gets substituted with more ______ groups.
Slightly Denser
PHYSICAL PROPERTIES: Phenols are usually ___________ than water
Colorless to Light Pink Solid or Liquid
PHYSICAL PROPERTIES: Color and States of Phenols
Strong Sharp Smell
PHYSICAL PROPERTIES: Smell of Phenols that are often described as medicinal or slightly burnt
Phenol
CHEMICAL PROPERTIES: They are MORE ACIDIC than regular alcohols
Phenol
CHEMICAL PROPERTIES: They can easily donate hydrogen ions because the benzene ring helps stabilize the negative charge that forms when the hydrogen is lost
Resonance Stabilization
CHEMICAL PROPERTIES: Stronger acid than alcohols due to the ______________ of its conjugate base
Conjugate Base
CHEMICAL PROPERTIES: Phenols are stronger acid than alcohols due to the resonance stabilization of its?
Phenol
CHEMICAL PROPERTIES: Forms esters with acids (e.g., esterification with acetic acid)
Strong Bases
CHEMICAL PROPERTIES: This acidity also allows phenols to REACTS WITH ___________ like sodium hydroxide which forms phenoxide ions which increase their solubility in water
Phenoxide Ions
CHEMICAL PROPERTIES: This acidity also allows phenols to REACTS WITH STRONG BASES like sodium hydroxide which forms ____________ which increase their solubility in water
Phenoxide Ions
CHEMICAL PROPERTIES: These ions increase solubility in water (Phenols)
Phenol
CHEMICAL PROPERTIES: They also undergo Oxidation forming compounds QUINONES
Quinones
CHEMICAL PROPERTIES: Phenols forms this and they are useful in biological activities and industry process
Highly Reactive
CHEMICAL PROPERTIES: Because of the (-OH) group of Phenols, the BENZENE RING in phenol, is __________, making it easier for phenols to undergo electrophilic substitution reaction (e.g., halogenation, nitration)
Electrophilic Substitution Reaction
CHEMICAL PROPERTIES: Because of the (-OH) group of Phenols, the BENZENE RING in phenol, is HIGHLY REACTIVE, making it easier for phenols to undergo _______________________
Electrophilic Substitution Reaction
CHEMICAL PROPERTIES: In Phenols, different atoms/group like HALOGENS OR NITROGEN GROUPS, can easily replace hydrogen atoms in the benzene ring, leading to the formation of important derivatives like antiseptics and disinfectants
Halogens, or Nitrogen Groups
CHEMICAL PROPERTIES: In Phenols, Electrophilic Substitution Reaction consists of different atoms/group like ___________ OR __________________, can easily replace hydrogen atoms in the benzene ring, leading to the formation of important derivatives like antiseptics and disinfectants
Epinephrine
4-[(1R)-1-HYDROXY-2-(METHYLAMINO)ETHYL]BENZENE-1,2-DIOL is also known as?
Epinephrine
EXAMPLES OF DRUGS CONTAINING PHENOLS: Used in ANAPHYLAXIS OR ASTHMA ATTACK
Anaphylaxis, or Asthma Attack
EXAMPLES OF DRUGS CONTAINING PHENOLS: Epinephrine are used in?
Allergic Reaction
Anaphylaxis is also known as?
Bacterial Growth
EXAMPLES OF DRUGS CONTAINING PHENOLS: Phenol PREVENTS ________ in epinephrine solutions keeping them safe for used overtime
Preservative
EXAMPLES OF DRUGS CONTAINING PHENOLS: Phenols acts as a ______ agent
Potent
EXAMPLES OF DRUGS CONTAINING PHENOLS: Epinephrinecan degrade when exposed oxygen/air, light, or heart. phenol can SLOW BREAKING DOWN, ensuring the drug to remain __________ for the long period of time
Contamination, and Degradation
EXAMPLES OF DRUGS CONTAINING PHENOLS: In short, phenols helps to keep epinephrine effective and safe by preventing __________ and slowing its ________
Propofol
2,6-DIISOPROPYLPHENOL is also known as?
Propofol
EXAMPLES OF DRUGS CONTAINING PHENOLS: Used as an ANESTHETIC AGENT (milk-like)
Emulsion
EXAMPLES OF DRUGS CONTAINING PHENOLS: Propofol is a _________ BASED DRUG (IN VIAL), meaning it can support bacterial growth
Antimicrobial
EXAMPLES OF DRUGS CONTAINING PHENOLS: Phenol acts as a ____________ AGENT, preventing contamination and making the drug safer for the repeated used
Antimicrobial
EXAMPLES OF DRUGS CONTAINING _____________ AGENT, preventing contamination and making the drug safer for the repeated used
Epinephrine
EXAMPLES OF DRUGS CONTAINING PHENOLS: In short, phenols helps to keep ___________ effective and safe by preventing contamination and slowing its degradation
Propofol
EXAMPLES OF DRUGS CONTAINING PHENOLS: In short, phenol in _____ keeps it free from harmful microbes and help it maintain its potency overtime