Concept 4.2: Carbon atoms can form diverse molecules by bonding to four other atoms
1/10
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
11 Terms
Electron configuration
How an atom’s electrons are arranged; the key to atomic bond types and numbers as part of their characteristics
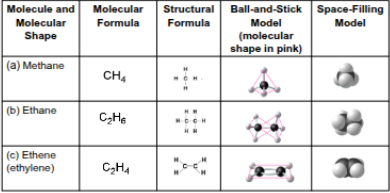
Carbon
An atom with four valence electrons that can form four covalent bonds
Molecules with multiple of these have a tetrahedral shape when joined to four other atoms
Two of these together in a double bond allow other atoms to stay in the same plane
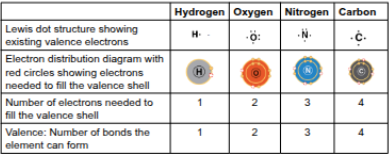
Valence
The number of unpaired electrons in the valence shell of an atom, determining the number of covalent bonds it can form
Carbon bonding partners
Most frequent are:
Hydrogen
Oxygen
Nitrogen
Carbon molecule examples
Carbon dioxide: CO2
Urea: CO(NH2)2
Estradiol and Testosterone
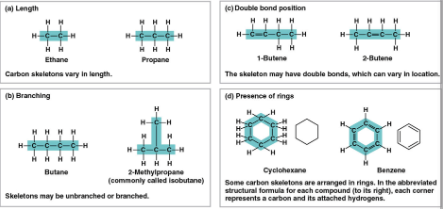
Carbon chains
The skeletons of most organic molecules that vary in length, shape, double bond position, branches, and ring presence
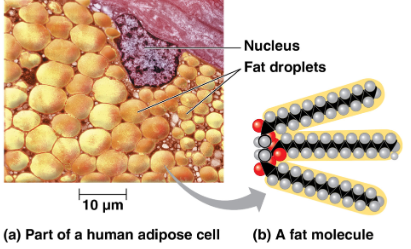
Hydrocarbons
Organic molecules consisting of only carbon and hydrogen
Many organic molecules, such as fats, have these as they can release a large amount of energy in some reactions
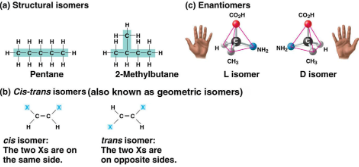
Isomers
Compounds with the same molecular formula (ratio) but different structures (arrangements) and properties, includes:
Structural: Different covalent bonds and arrangements
Cis-trans: Differing spatial arrangements, same covalent bonds
Enantiomers: Mirror images
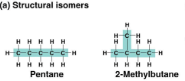
Structural isomers
Isomers with different covalent bonds and arrangements
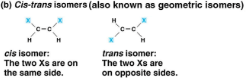
Cis-trans isomers
Isomers with the same covalent bonds but differing spatial arragnements
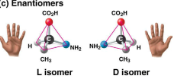
Enantiomers
Isomers that are mirror images of each other
Important in the pharmaceutical industry, as each pair may have different effects or not be effective at all, demonstrating molecular sensitivity
Ibuprofen’s and albuterol’s versions are ineffective