sliding filament theory and excitation contraction coupling
1/12
Earn XP
Description and Tags
week 4 unit 3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
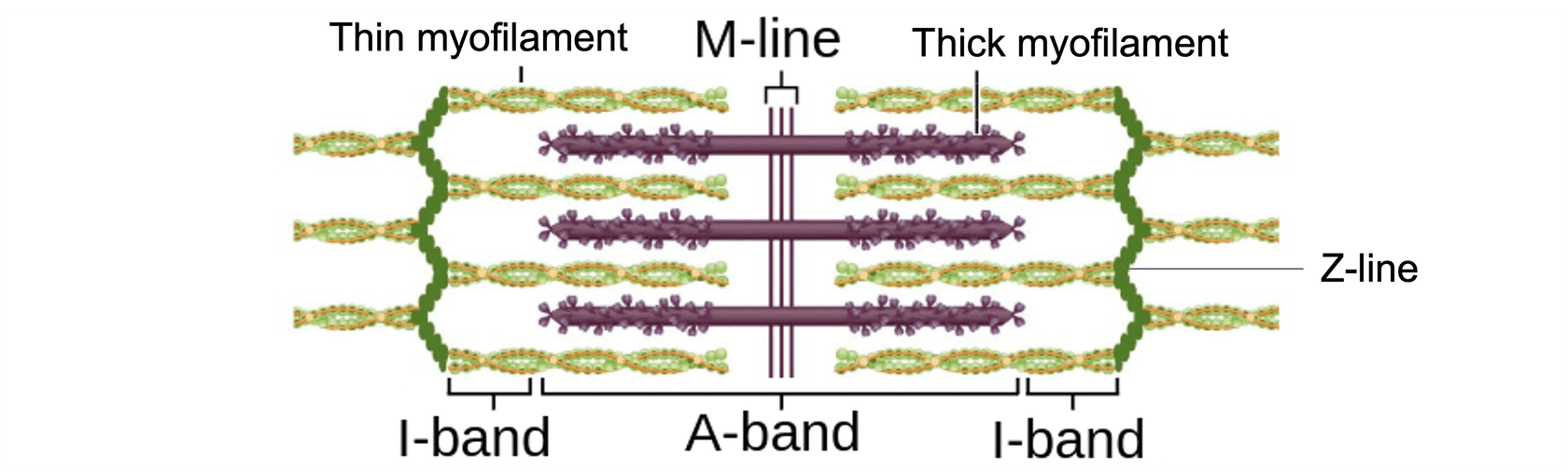
sliding filament theory
shortening of sarcomere during contraction
increase of overlap due to thin myofilament silding over thick myofilament
thus moving towards the m-line
sliding event occurs due to cross bridge
what is cross bridge
formed between actin in the thin myofilament and myosin in thick myofilament
what is the power stroke and what happens after
head of the myosin molecule binds to the myosin binding site on actin
upon binding, head changes shape, propelling the thin filament towards the m-line
following a power stroke, thin filament slides past myosin, moving closer to the m-line and pulling the z-lines/discs closer, shortening the sarcomere
order of sliding filaments using crowd surfing analolgy
contraction is triggered
myosin head binds to actin- cross bridge is formed (hand of crowd catch the board)
myosin head changes shape - power stroke occurs (hand propel the board + surfer forward thru wrist motion)
thin myofilament slides past thick myofilament, moving towards the m-line (surfer+board move only in one direction)
z-discs/lines come closer together
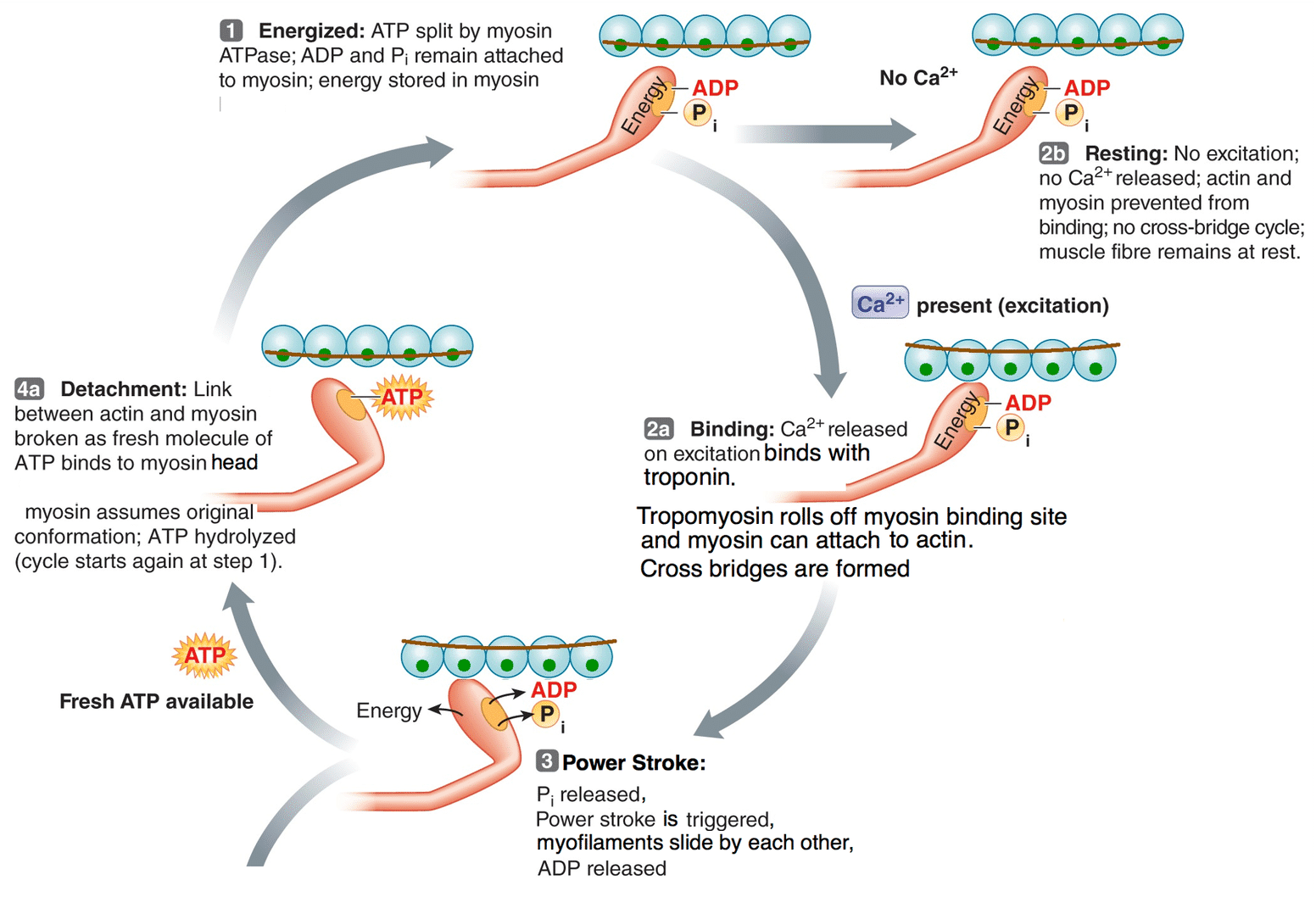
excitation-contraction coupling
occurs when an action potential on the sarcolemma leads release of calcium ions from the sarcoplasmic reticulum (SR) leading to cross bridge formation, power-stroke and muscle contraction
explain 1. energized state (atp hydrolysis)
ATP binds to ATPase binding site on myosin head
ATPase enzyme breaks down (or hydrolyzes) ATP into ADP and inorganic phosphate (Pi)
this process releases energy that moves the myosin head into position to bind to actin (it activates/energizes hyosin head)
ADP and Pi remain in the ATPase site during the energized state of the myosin head

presence of calcium
if an AP was generated in muscle cell, calcium is release from the sr
which binds to troponin c
troponin c moves to tromyosin, exposing the myosin binding site on actin
if the myosin head is activated, myosin binding site on actin is exposed, then cross bridge forms and contraction occurs
absence of calcium
if no AP generated in muscle cell, calcium wont be released from the sr
the myosin binding site on actin wont be exposed and cross bridge wont form
therefore no contraction
power stroke process
involves change in conformation of the myosin head
that change in conformation occurs when the Pi is released from ATPase binding site in myosin
As Pi moves out, the myosin head pulls on actin
directs the thin myofilament towards the m-line and shortening of the sarcomere
after the power stroke, ADP is also released from ATPase site on myosin
ATP binding (DETACHMENT)
when the ATPase site on myosin is empty, a new ATP molecule binds
the binding of atp leads to another change in the myosin head
it detaches from actin and resumes low energy conformation
as soon as ATPase breaks apart the ATP (it hydrolyzes it), the cycle resumes starting at energized state
rigor mortis
stiffening of muscles after death (3-4 hours after)
no oxygen =no ATP - in the cell, mitochondria need oxygen to produce ATP
no atp=no detachment - since atp causes the actin-myosin cross bridge to break by binding to ATPase site on myosin, no ATP means actin and myosin cannot detach
no detachment=actin and myosin remain fused
no atp=no calcium back in sr - if theres no atp, calcium atpase on sr cant function bc it need substrate
no calcium back in sr=more cross bridges form - since now calcium is available in the cytoplasm, it binds to troponin c, exposing the myosin binding site and allowing more cross bridges to form
more cross bridges form + actin-myosin remaining fused = muscle is fused or in rigor
can the muscle remain permanently in rigor mortis?
no bc muscle begins to decompose over time meaning cross bridges begin to fall apart as the contractile proteins are denatured during the decomposition process. at higher temps, rigor mortis occurs faster. humidity, bacteria age, body fat, etc will impact rigor mortis