Molecular & Cell Bio. - Exam 4/Quiz 2 - Protein Quality Control
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
what is the native structure of a protein?
the 3D shape of a protein that is specified by the amino acid sequence
what are molecular chaperones/co-chaperones? what are their functions? provide an example
proteins that assist in the folding of other proteins
- facilitate co- and post-translational folding
- assist in assembly and disassembly of macromolecular complexes
- suppress misfolding and aggregation of unfolded polypeptides
ex: heat shock proteins
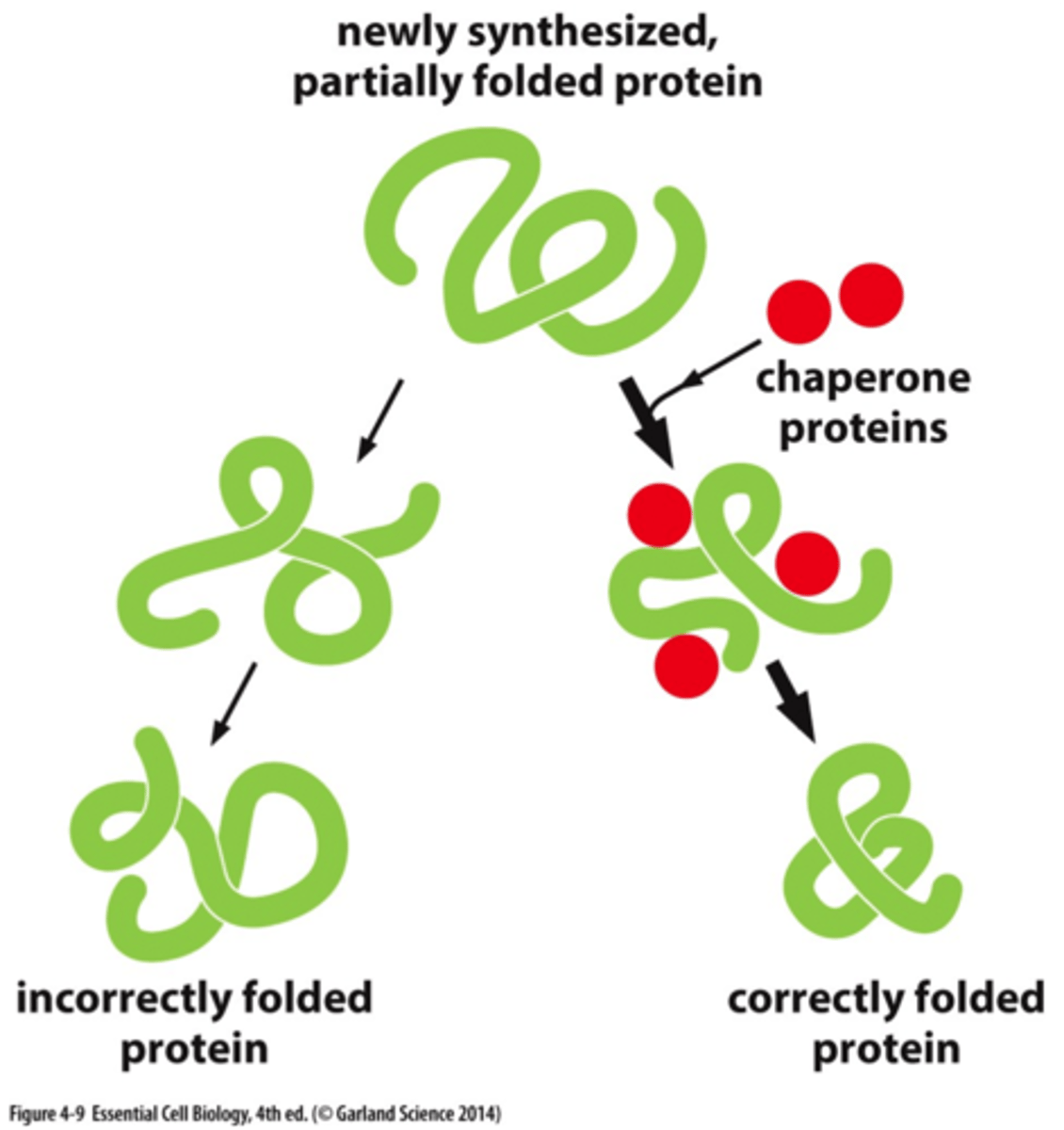
what happens if molecular chaperones are inhibited?
misfolded proteins accumulate
- oligomers, amorphous aggregates, and amyloid fibrils
- some protein aggregations can be toxic!
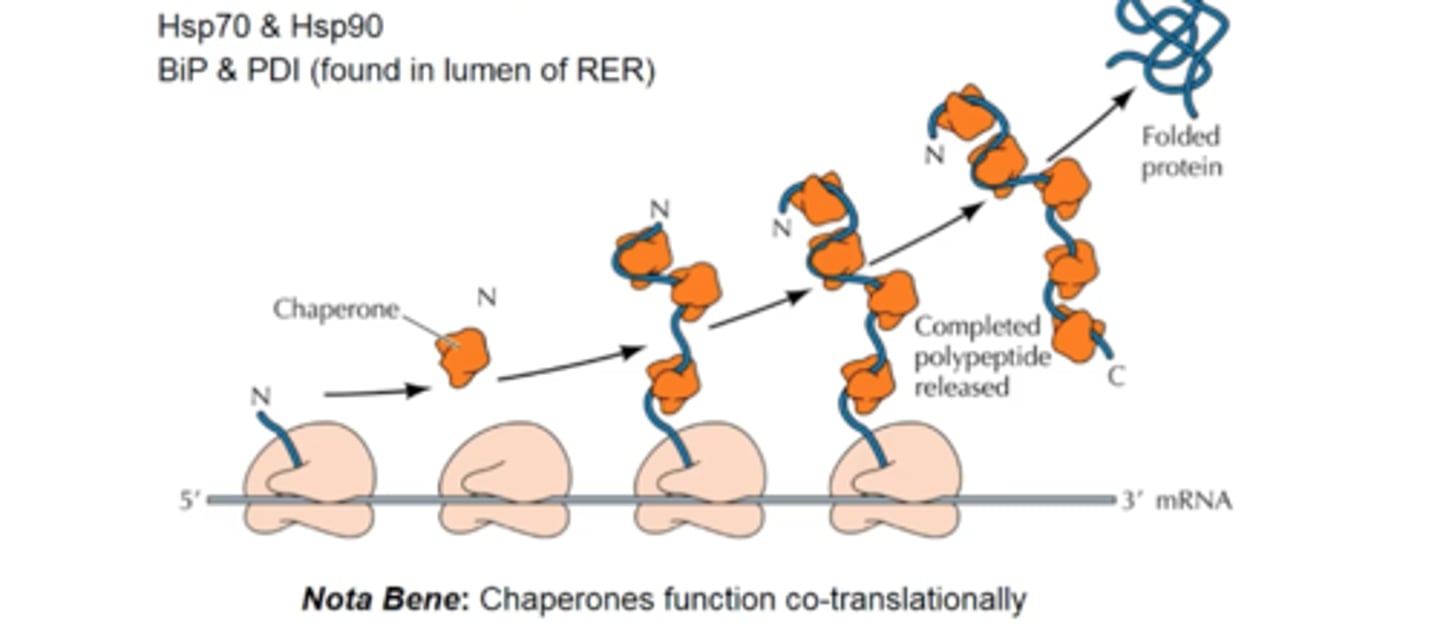
describe the action of chaperone proteins during translation
bind to the N-terminus of the growing peptide chain, stabilizing it in an unfolded configuration until peptide synthesis is complete
- the completed protein is then released from the ribosome and is able to fold into its correct 3D conformation
a partially unfolded polypeptide is transported from the cytosol to the _____________
mitochondria
- peptide is stabilized by chaperones (facilitate transport)
list the protein/functions that are associated with the chaperone HSPs Hsp60, Hsp70, Hsp90, and Hsp100
- Hsp60 (TriC, GroEL): ATPase folding
- Hsp70 (DnaK, BiP): ATPase stabilization of extended chains, membrane translocation, and regulation of the heat shock response
- Hsp90 ATPase (A, B, Grp78): binding and stabilization/regulation of steroid Rs and protein kinases
- Hsp100 ATPase (Clp): thermotolerance, proteolysis, resolubilization of aggregates
most proteins leaving the ribosome are met by ___________ chaperones; how do these chaperones interact with the peptide?
Hsp70
- interacts with short lengths of hydrophobic amino acids (typically 7 residues)
- frequently function with co-chaperones, such as Hsp40 (J protein)
what is the function of Hsp40 (J protein)?
co-chaperone
- targets substrates to Hsp70 and stimulates ATP hydrolysis
- transiently associates with unfolded protein substrates for delivery to Hsp70
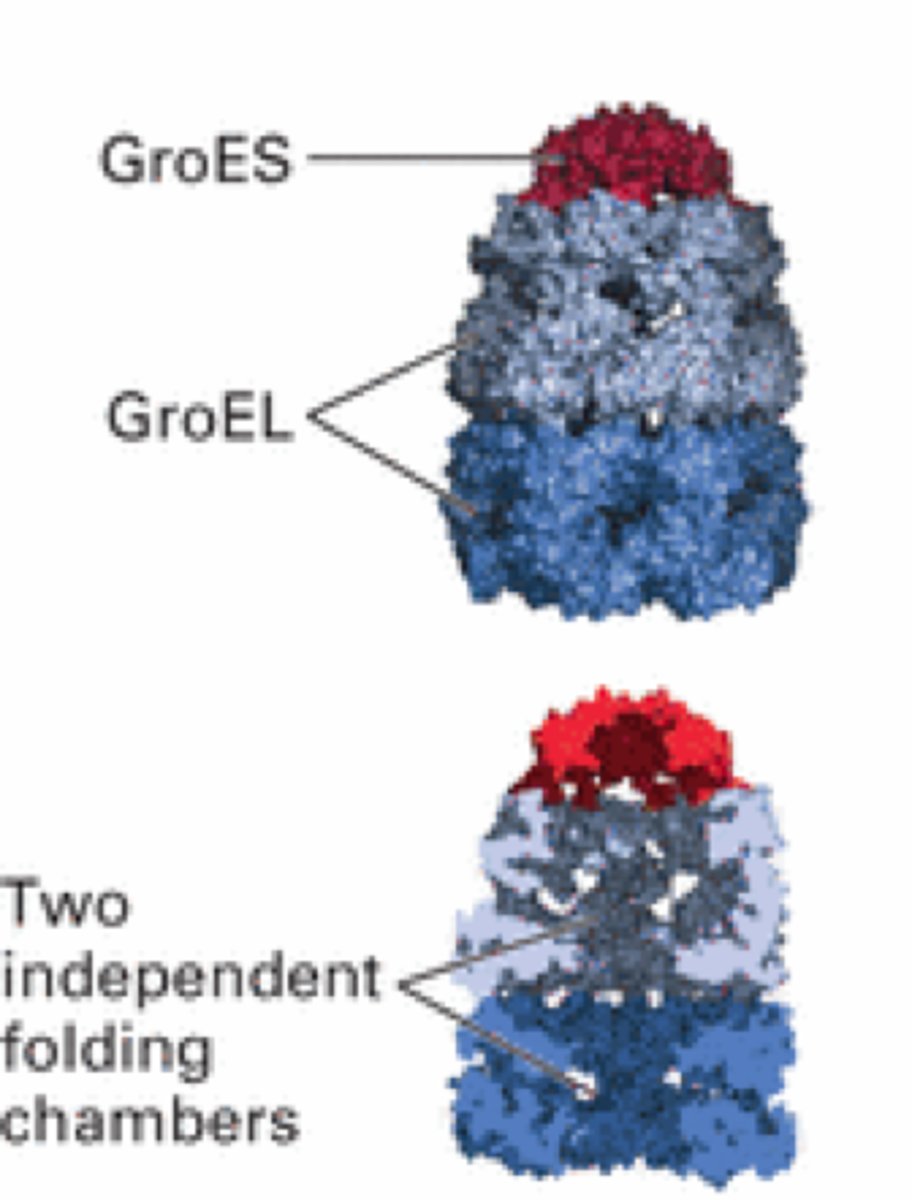
the type I Hsp60 chaperone protein present in bacteria is called ____________; describe its function
GroEL
- coordinates with the Hsp10 bacterial co-chaperone GroES
- prevent misfolding of proteins during times of stress, such as high heat
the type II Hsp60 chaperone protein present in eukaryotes/archaea is called ____________; describe its function
T-complex protein ring complex (TRiC)
- constitutively expressed
- interacts with ~10% of newly synthesized proteins (actin, tubulin, cyclin E, cdc20, VHL, etc.)
- binds co-translationally to nascent chains as they emerge from the ribosomes
describe the sequential actions of Hsp70/60 chaperones
- Hsp70 chaperones bind and stabilize the unfolded polypeptide DURING translation
- the unfolded peptide is then transferred to Hsp60 chaperones, which fold the protein
T/F: ATP hydrolysis is required for release of the unfolded peptide from Hsp70 AND for folding by Hsp60
TRUE
- needed for both processes
what is the function of Hsp90 chaperones?
dimeric; acts as part of a dynamic complex with other chaperones/co-chaperones
- stabilizes proteins against heat stress and aids in protein degradation
- requires cooperation with Hsp70 and its cofactor (Hsp40)
what are the isoforms of Hsp90 chaperones?
- Hsp90α: inducible expression
- Hsp90β: constitutively expressed
BOTH are cytoplasmic!
under stressful conditions, Hsp90 protects ______________
oncoproteins
- Hsp90 is upregulated in cancers, which contributes to proliferation and decreased apoptosis
T/F: proteins misfolding can occur spontaneously
TRUE
- can also be caused by genetic mutations!
what are amyloids?
aggregates of misfolded protein
- implicated in neurodegenerative disorders like Alzheimer's and Parkinson's disease
what is the proteolytic system, and how does it prevent cell damage by unfolded proteins?
molecular mechanism to avoid any cellular damage caused by the accumulation of unfolded proteins
- mechanisms include ubiquitin-proteosome degradation system (UPS) and autophagy
what are the 4 primary disease classes that can be caused by excessive protein misfolding?
- disordered protein aggregate → cataracts, muscular dystrophy, Huntington's
- amyloids → Alzheimer's, Parkinson's, prion's disease, cardiac amyloidosis
- enhanced degradation → emphysema, phenylketonuria (PKU)
- trafficking defects → Tay Sach's, CF
mutations in the precursor protein for __________ are associated with Alzheimer's
Aβ
- β-amyloid precursor protein, APP
describe the general process that is believed to cause Alzheimer's
1) stress responses or aging causes oxidative stress
2) oxidative stress causes mitochondrial dysfunction and altered synaptic transmission
3) this causes impaired function of chaperones and PQC, leading to misfolded proteins
4) aggregation of Aβ-aggregates of misfolded proteins
steps 1, 2, and 4 may all directly cause Alzheimer's
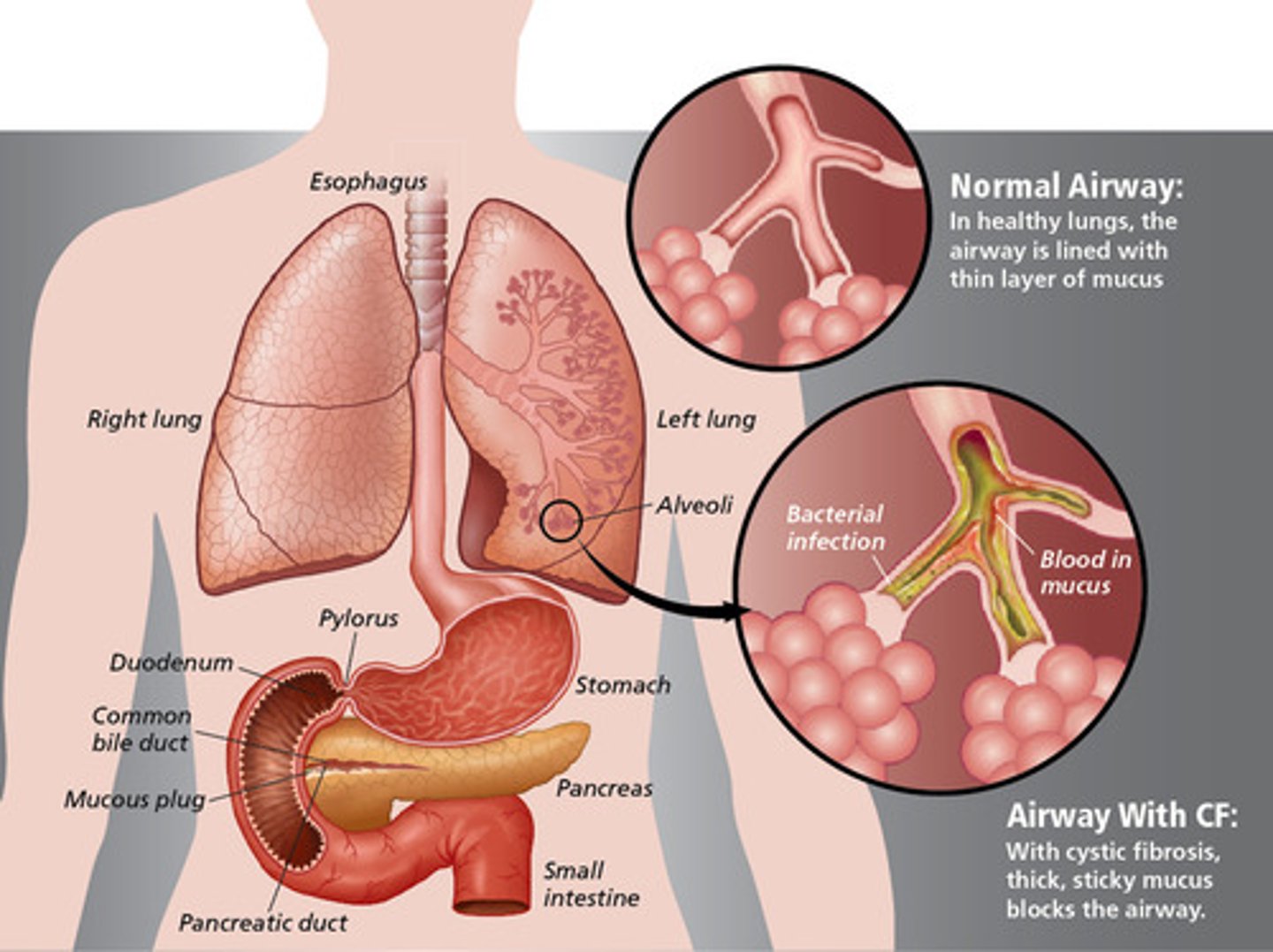
what mutation is present in >70% of CF patients? how does this lead to disease?
DF508 mutation of the CF transmembrane R (CFTR)
- CFTR is a Cl- channel on the apical surface of epithelial cells lining the lung airways
- CFTR DF508 misfolds in the ER
- leads to a buildup of IC Cl- ions