Unit 4: Light, Energy & Electron Configuration
1/24
Earn XP
Description and Tags
Unit 4 | Chemistry | Mrs. Macurdy
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
25 Terms
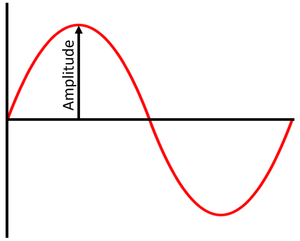
Amplitude
Measure of the height of a wave from the mid-line to the top of a crest or the depth of a trough from the mid-line.
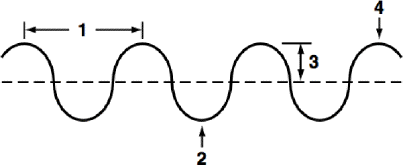
What is the term for number 2?
Trough: lowest point or valley in a wavelength
Light is an…
…electromagnetic wave
Photon
A little packet of energy that can carry electromagnetic radiation
Wavelength
Distance between 2 neighboring crests or 2 neighboring troughs
Frequency
Number of waves that pass a certain point per second
As frequency increases…
…Wavelength decreases/shortens (vice versa)
Hertz (Hz)
Frequency Unit
Ground State
Lowest energy state of an electron in an atom
Excited State
Highest energy state; when an electron absorbs energy
What form of energy results from an electron going to ground state from excited state?
Light (Light energy is released often in the form of a photon)
R.O.Y.G.B.I.V is part of what portion on the EM spectrum?
Visible Light
C=(λ)(v)
Speed of light=Wavelength x Frequency
E=(h)(v)
Energy=Planck’s Constant x Frequency
If only the wavelength is known, what formula do we use to solve for energy? (Yes, energy can be calculated)
E=(h)(v)/λ
Which element has the most EN(electronegativity)?
Fluorine
Ion
Any atom that has a +/- charge
Ionic Radius
Radius of a cation or anion; neutral atoms ionized result in change of size
What unit is ionization energies measured in?
KJ/mol (Kilojoules per mole)
What is the electron configuration for Cr (Chromium)?
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵ 4s¹
What is the electron configuration for Ag (Silver)?
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 4d¹⁰ 5s¹
What is the electron configuration for Cu (Copper)?
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s¹
What is the electron configuration for Mo (Molybdenum)?
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d⁵ 5s¹
What is the noble gas configuration for Se (Selenium)?
[Ar] 3d¹⁰ 4s² 4p⁴
What is the final configuration for P (Phosphorus)?
3p³