H NMR Values
1/24
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
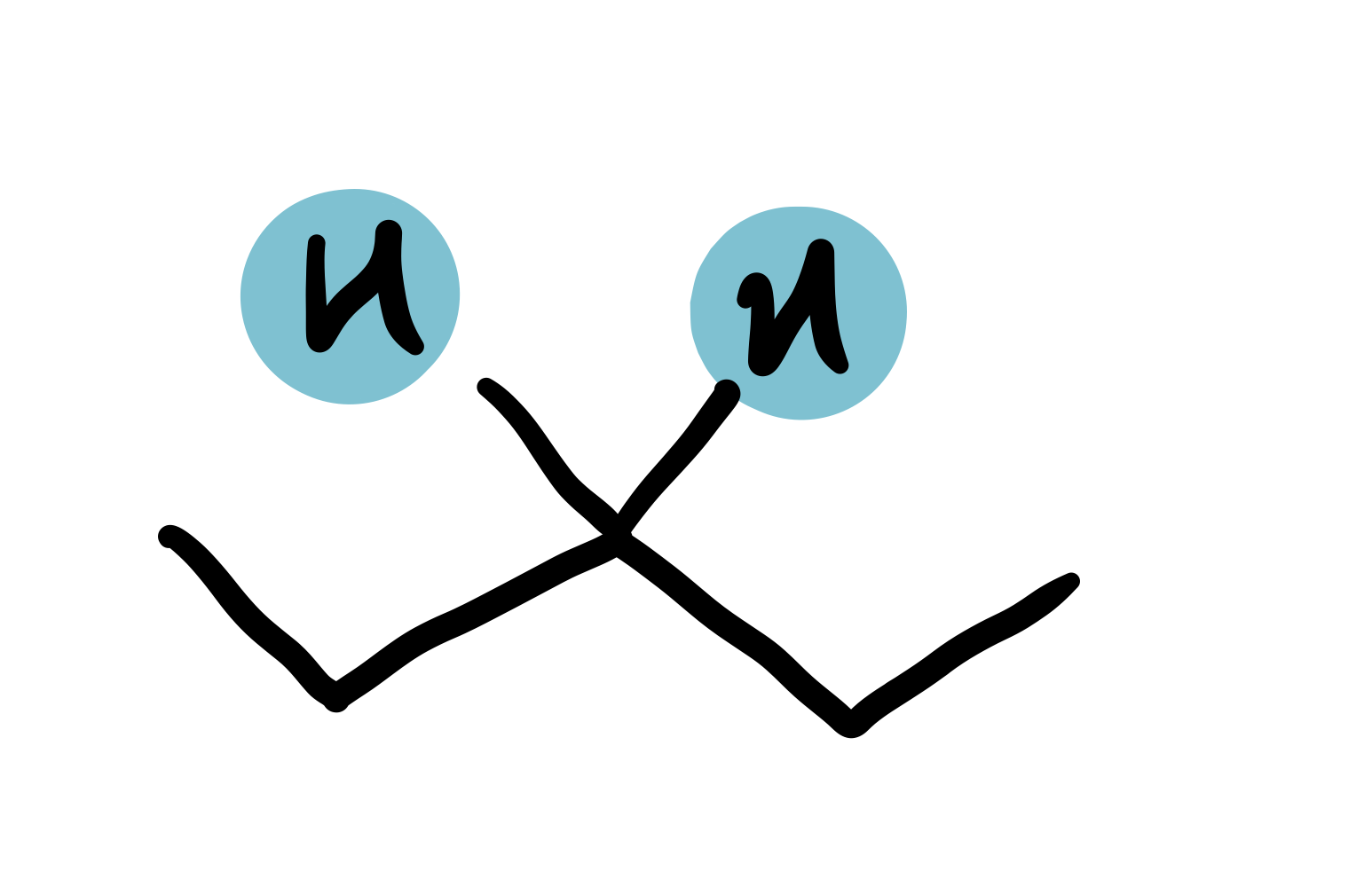
Middle of C chain
1.5-1.75
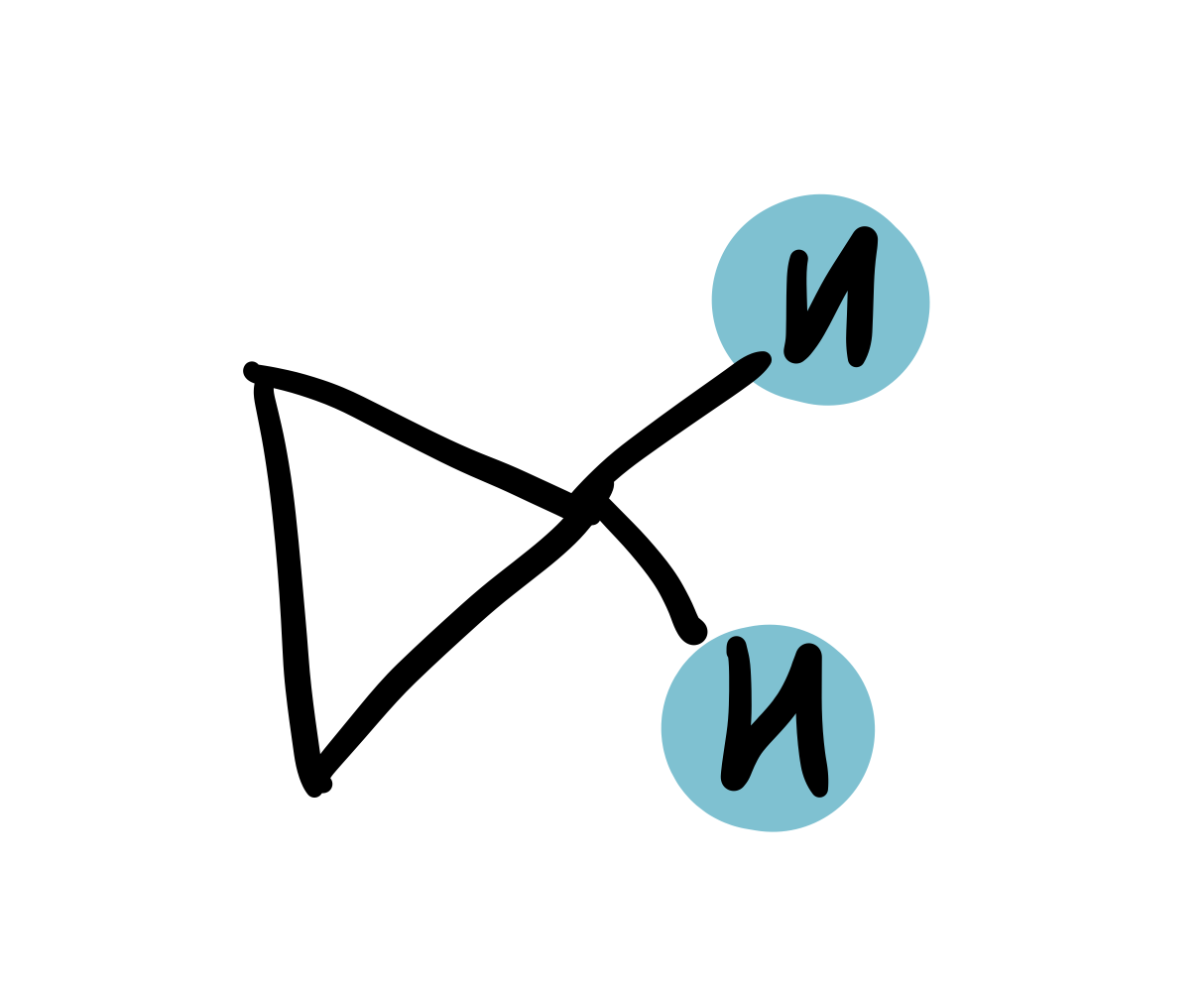
cyclopropane
0.25-0.5
amine (H-N) / alcohol
0.5-4.5 (could potentially appear anywhere)
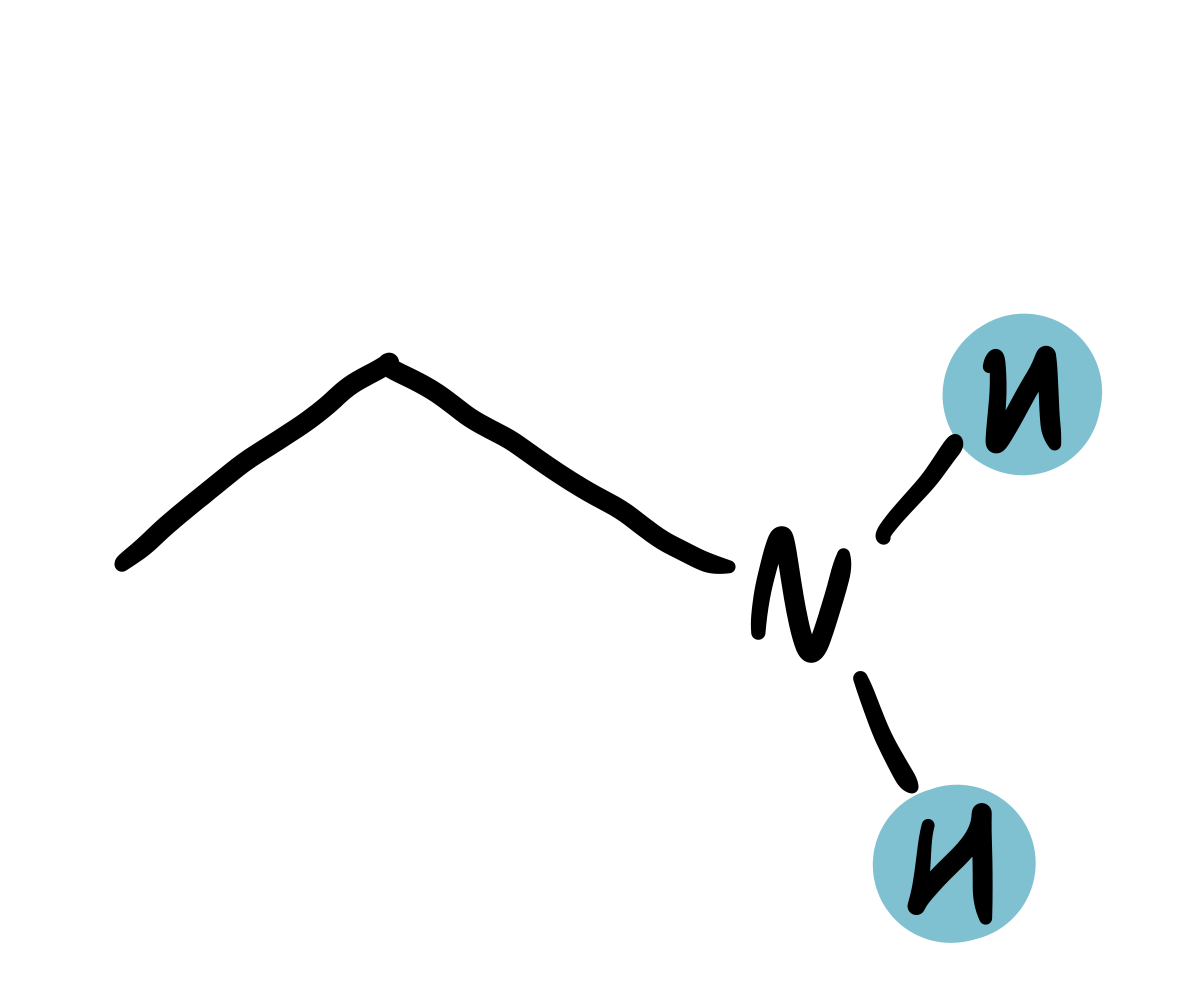
amine-adjacent (H-C-N)
2.25-2.6
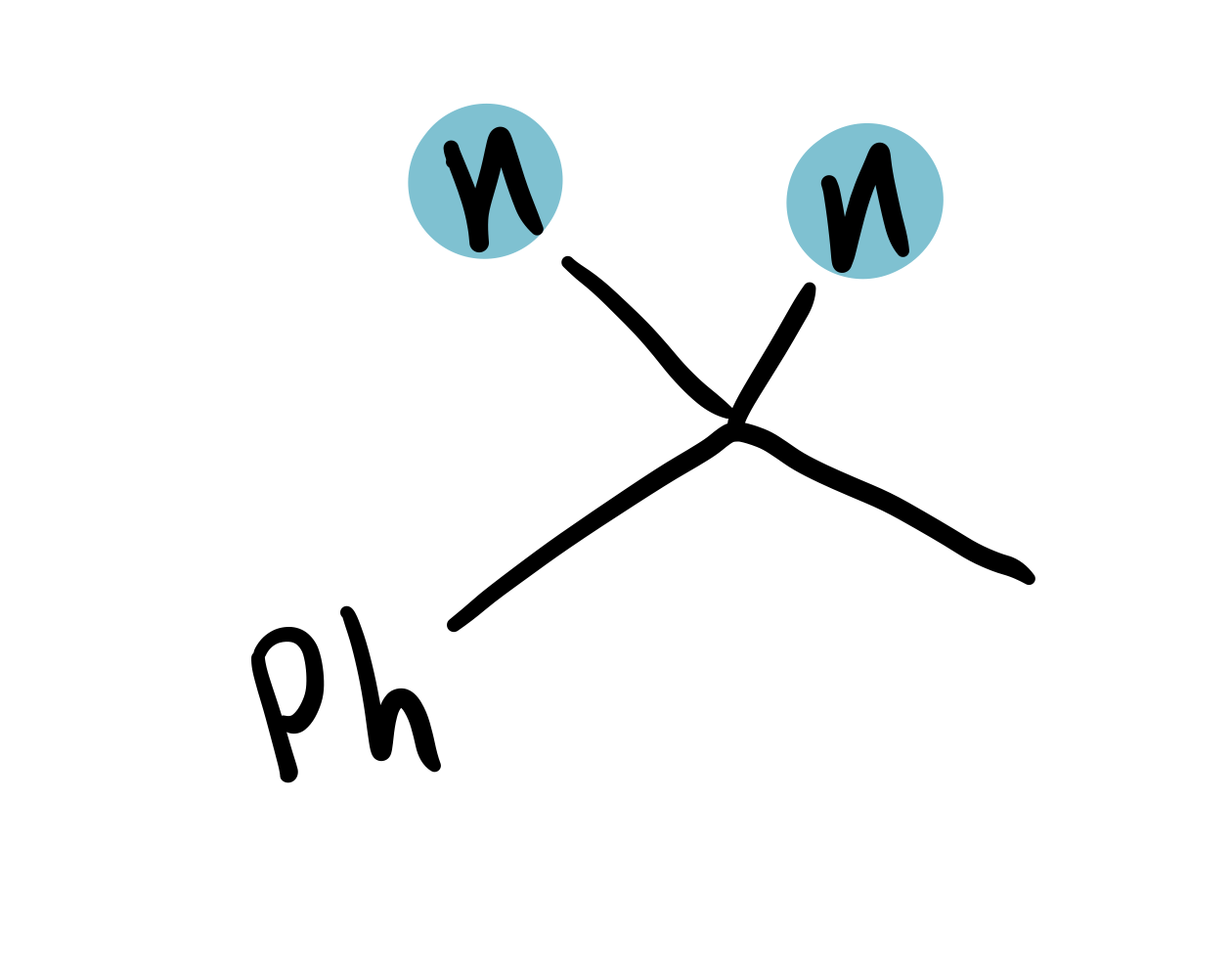
C chain attached to Ph
2.4-2.8
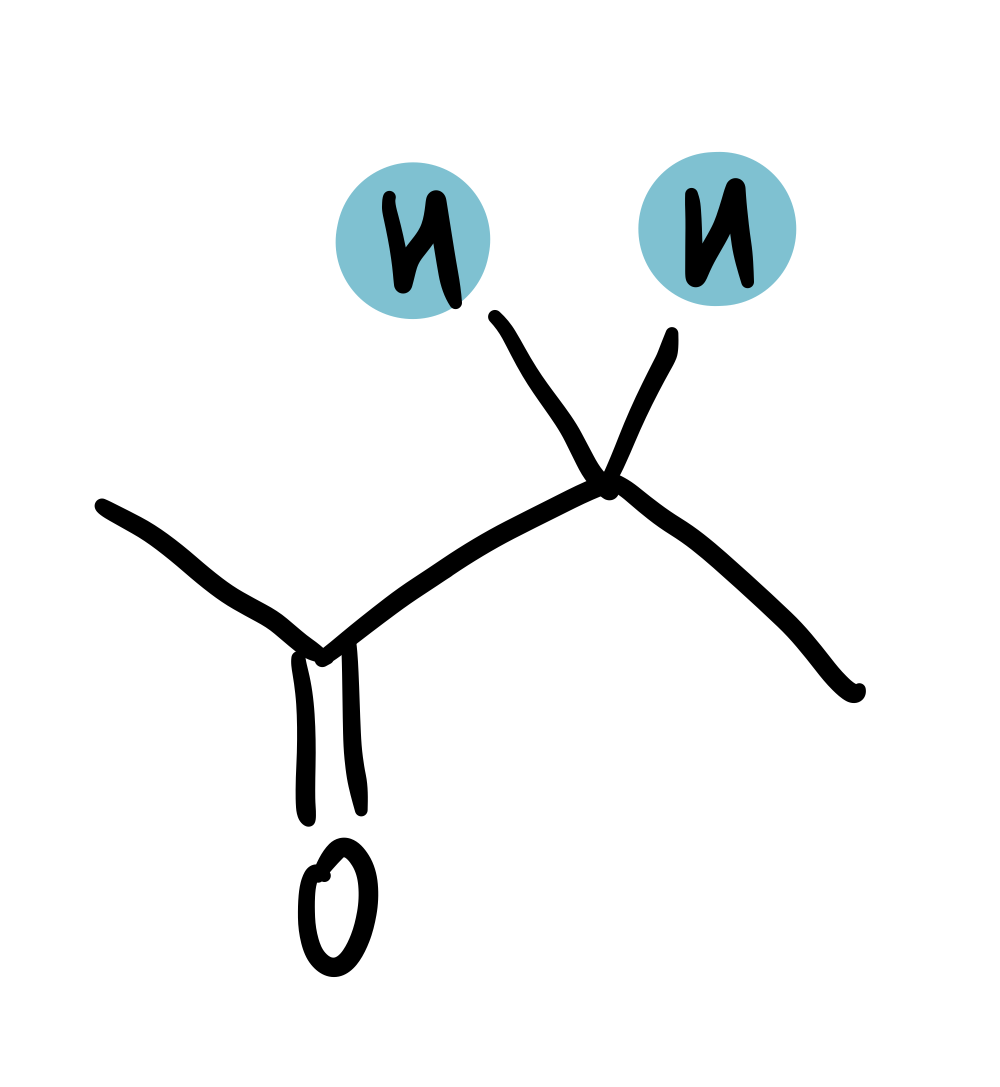
Ketone-adjacent
2-2.75
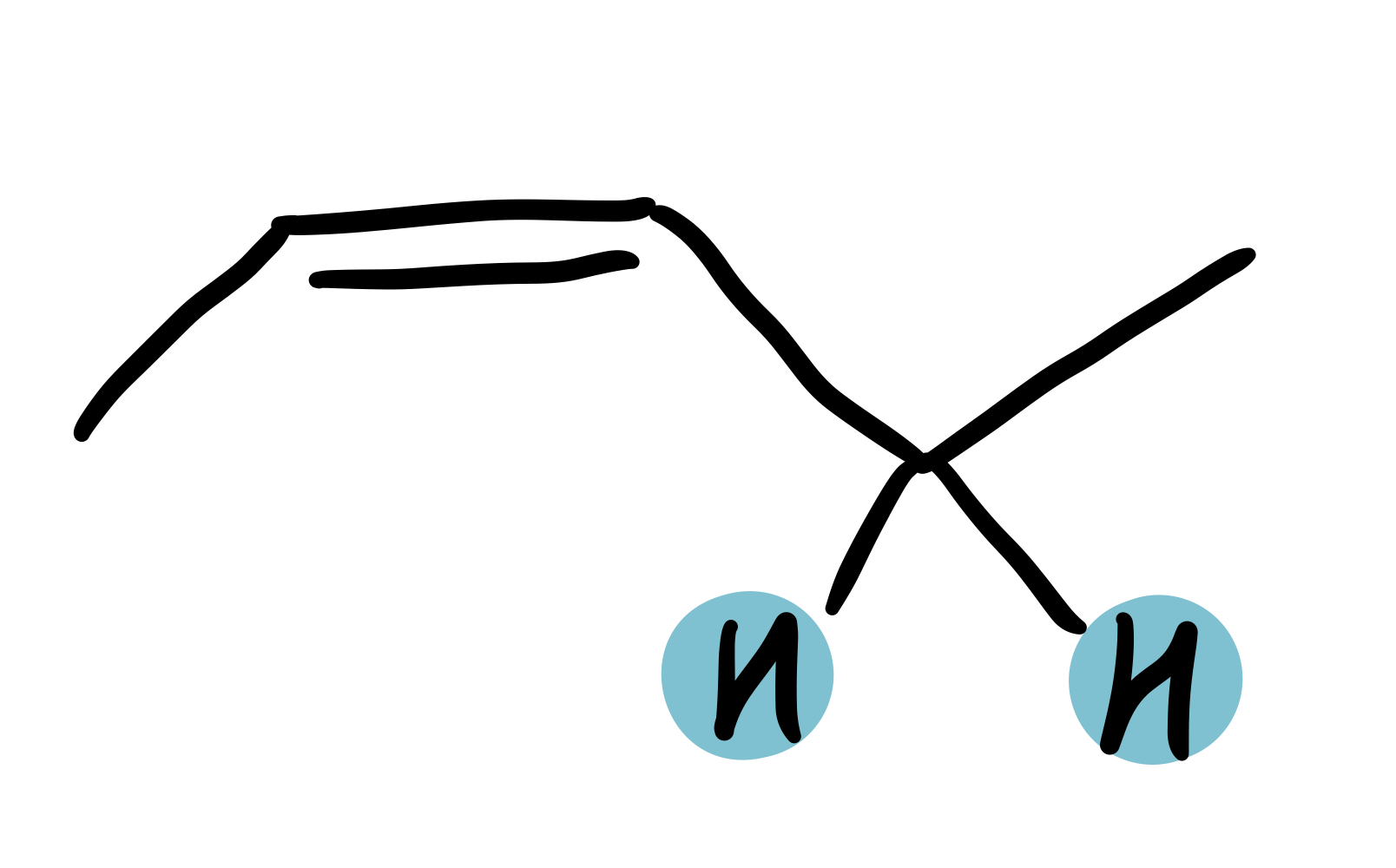
alkene-adjacent
1.8-2.25
How does being on a Me affect the shift?
-0.5

alkyne
1.75-2.75
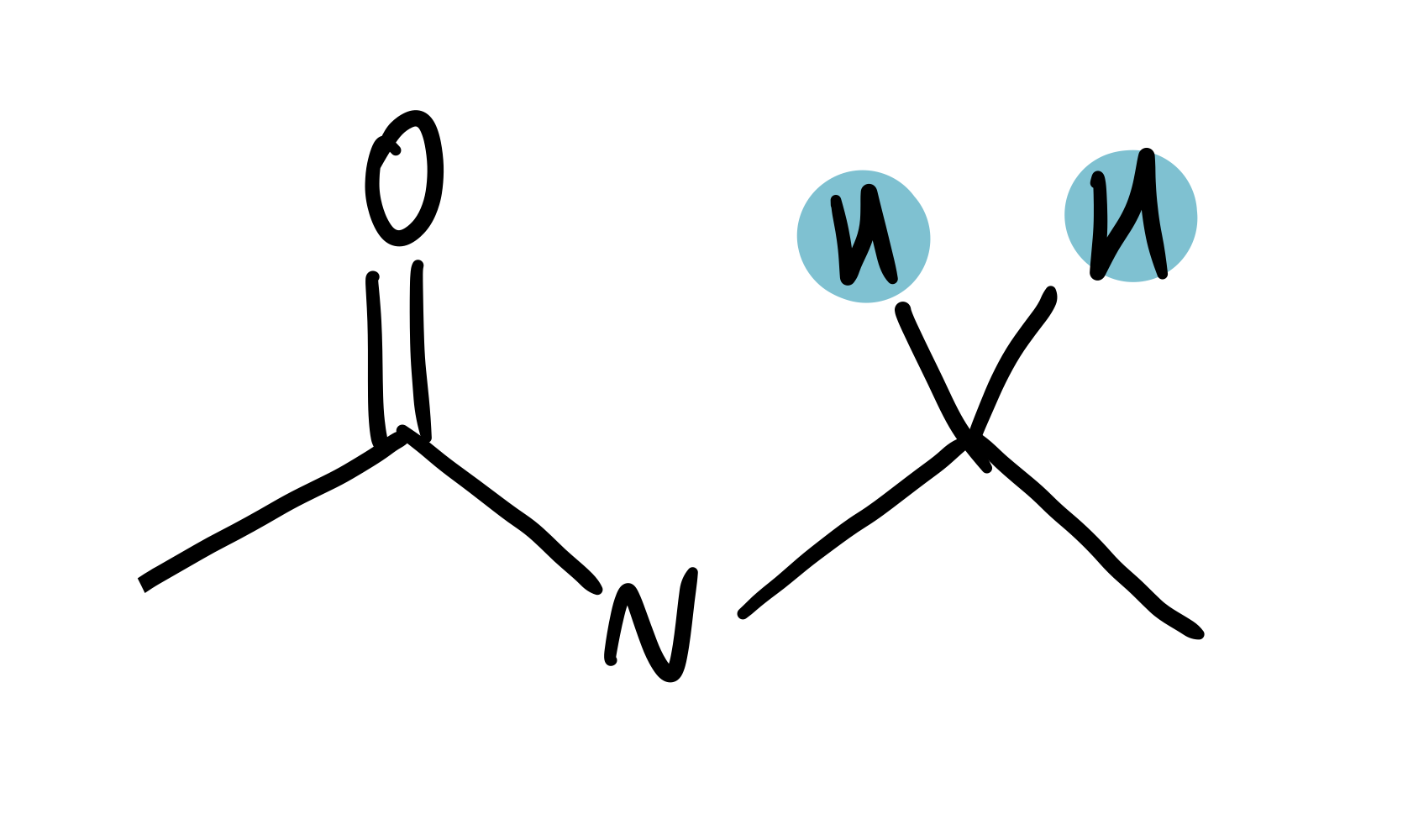
amide-adjacent
3.25-3.6
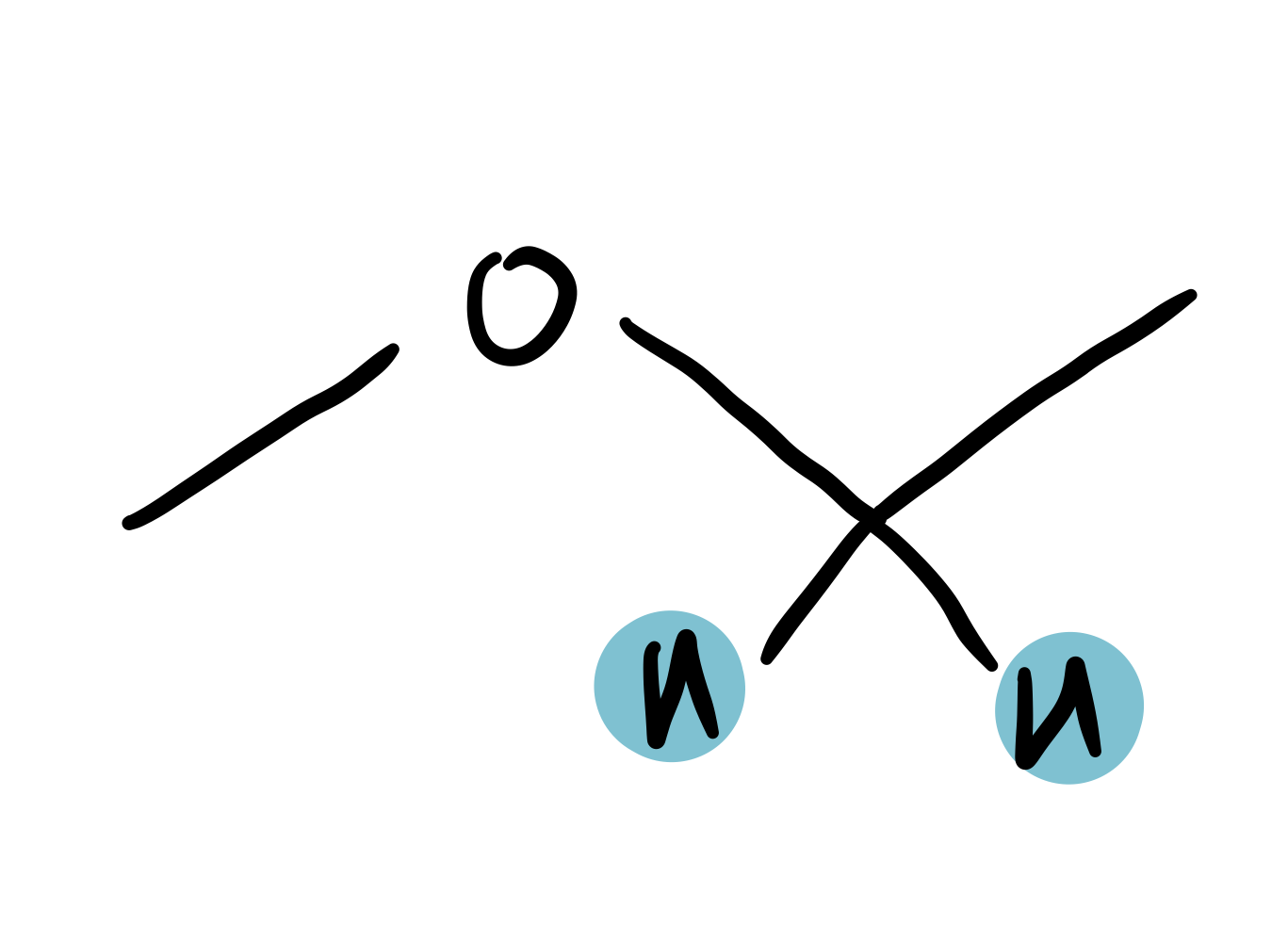
ether
3.4-3.8
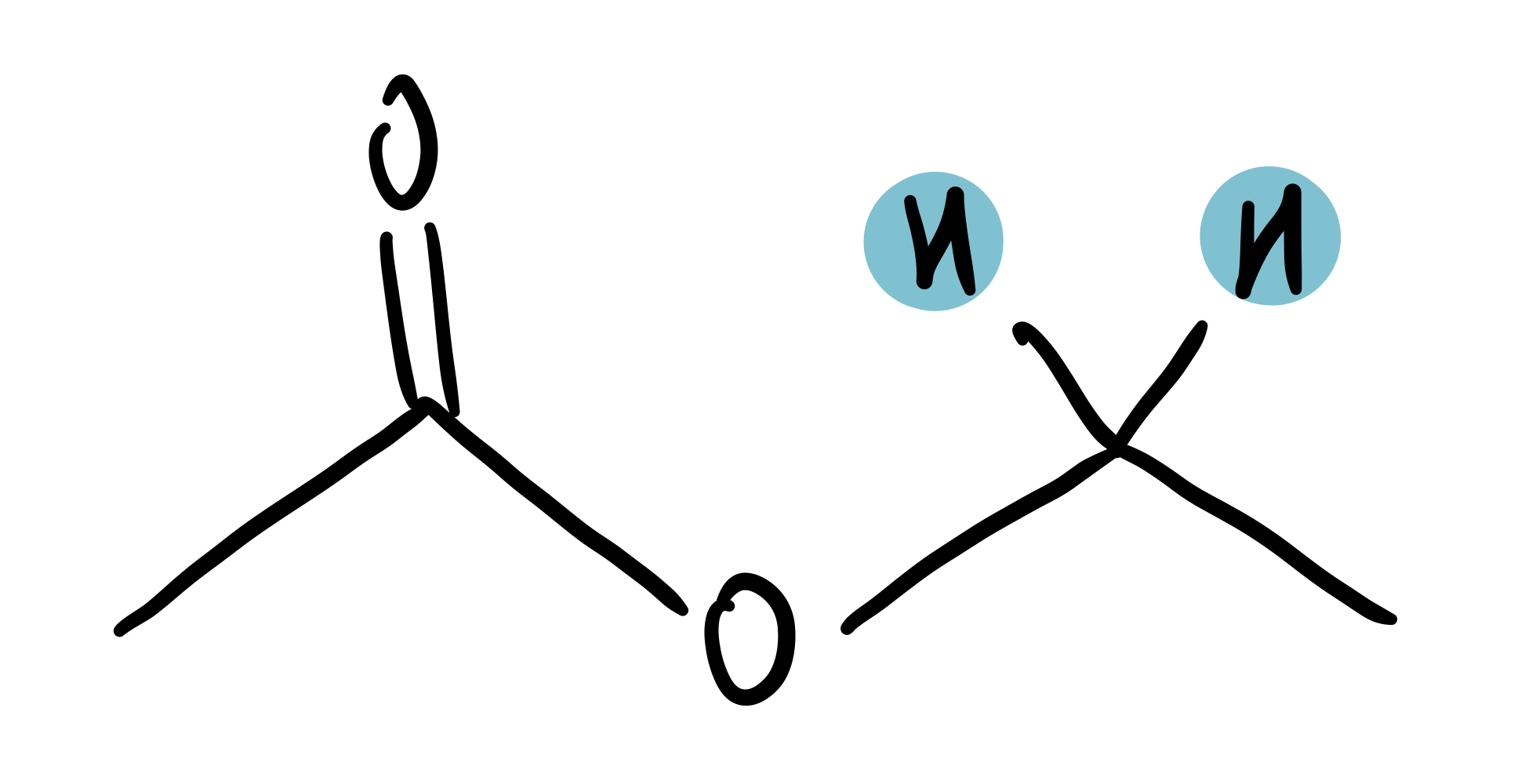
ester
4-4.5
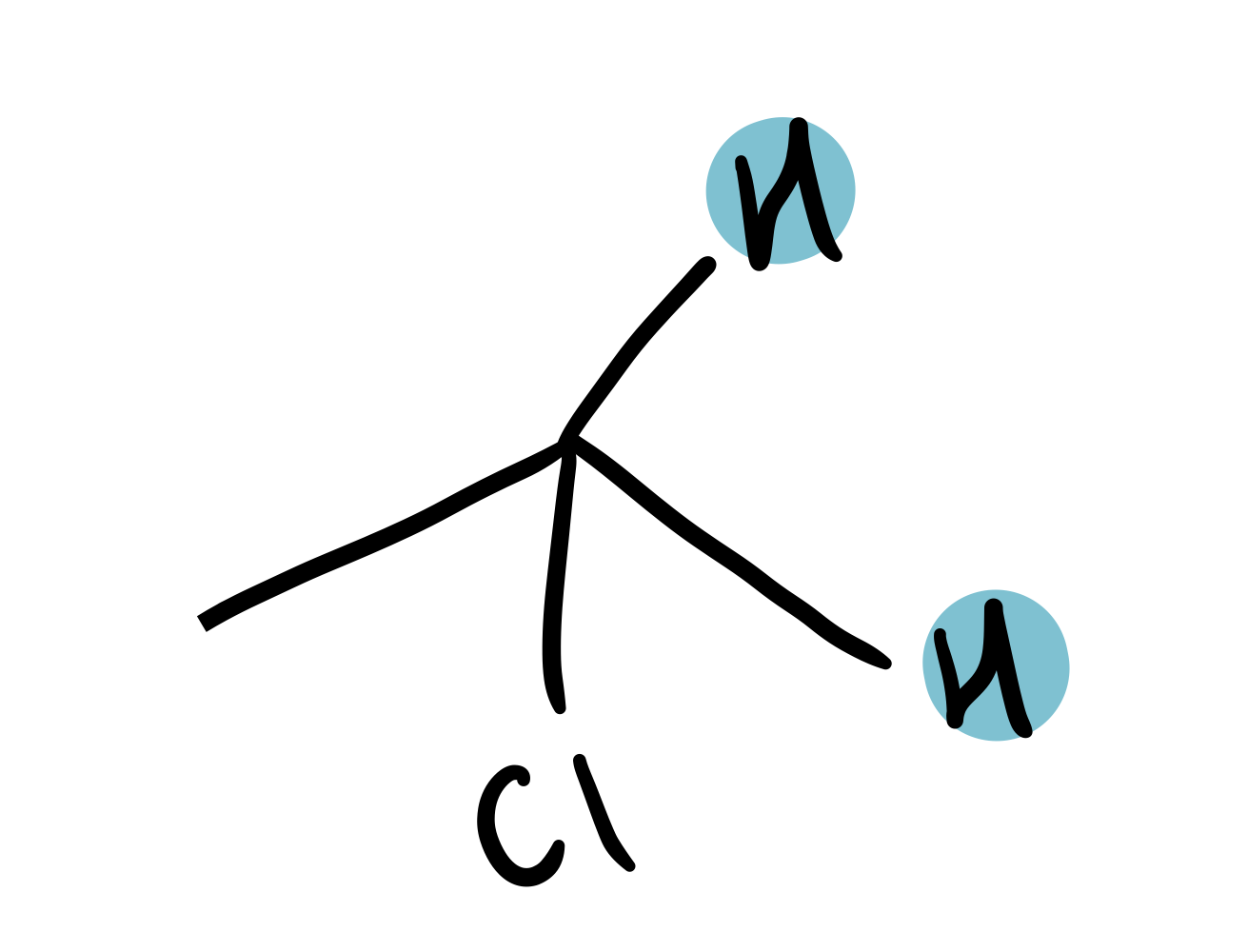
CH₂Cl
3.6-4.1
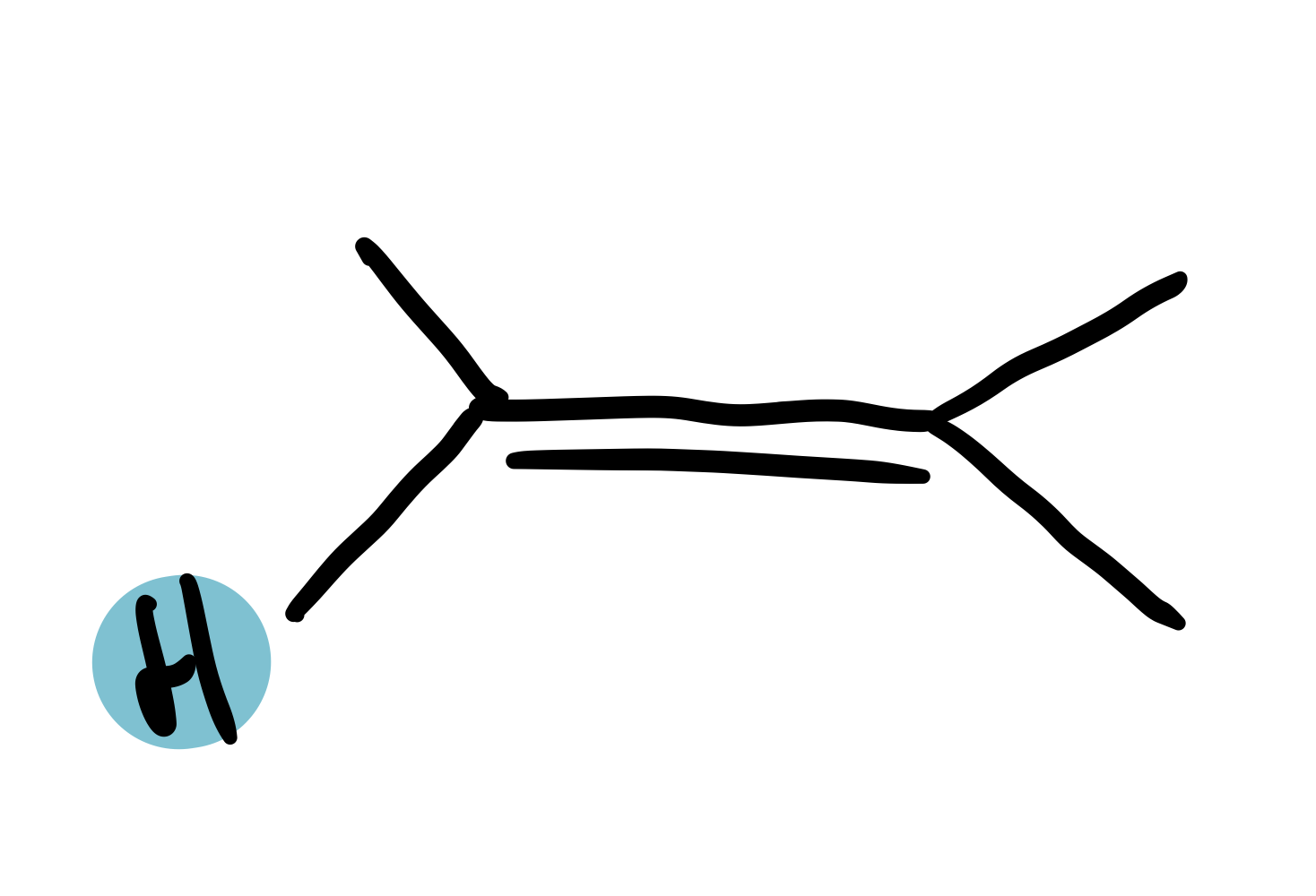
alkene
4.5-6
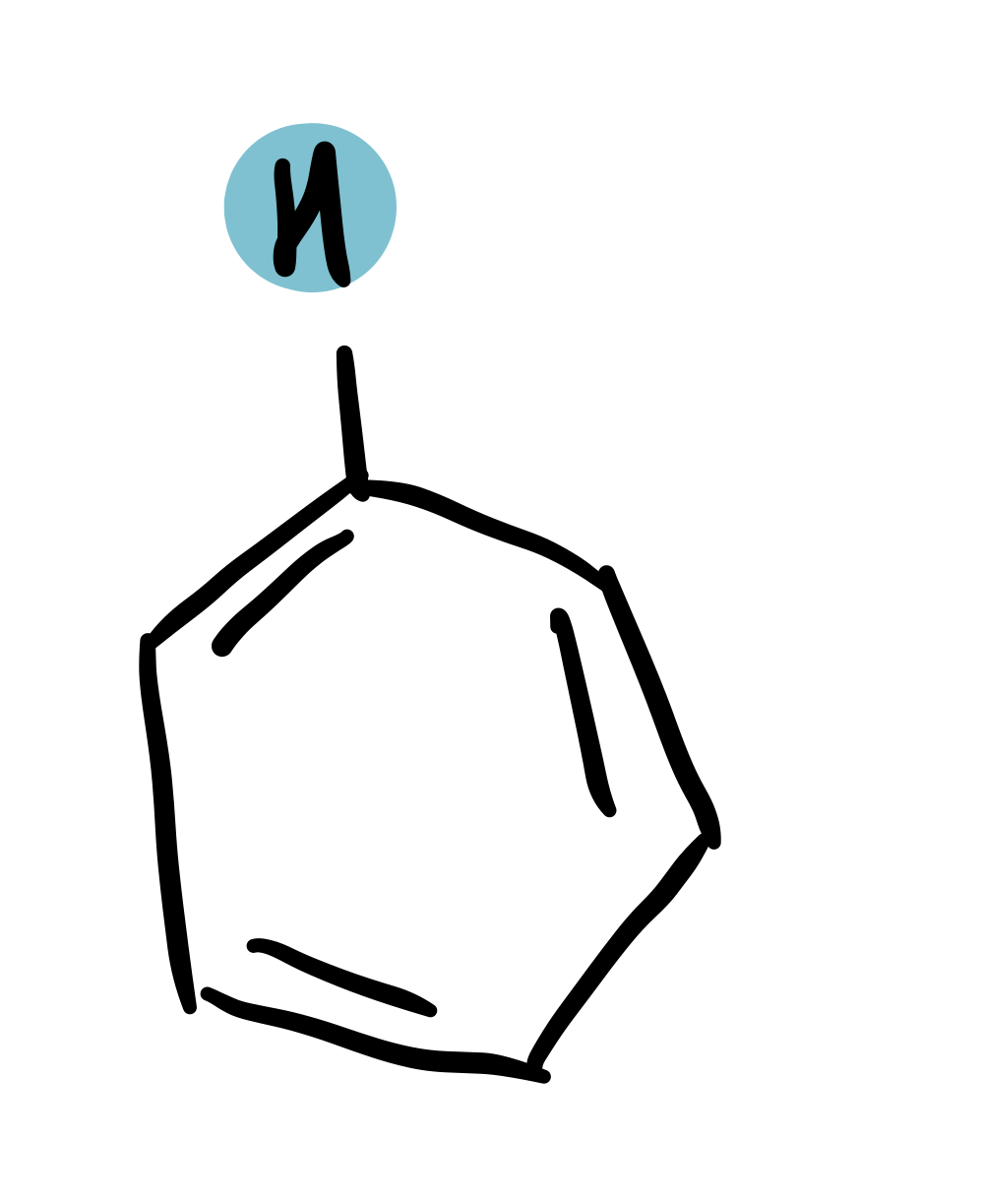
Benzene
6-9
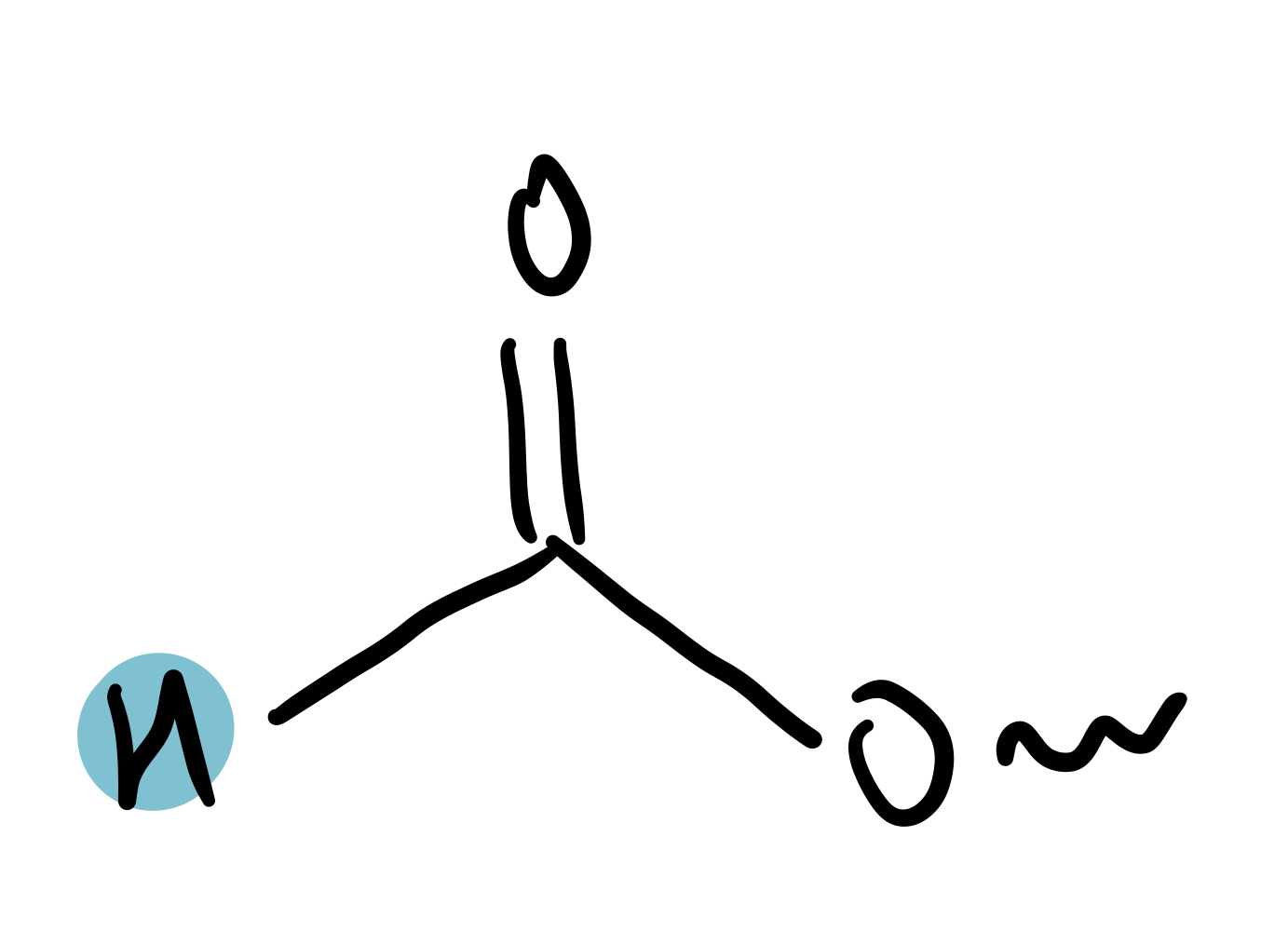
-methanoate (HCOO-)
8-8.4
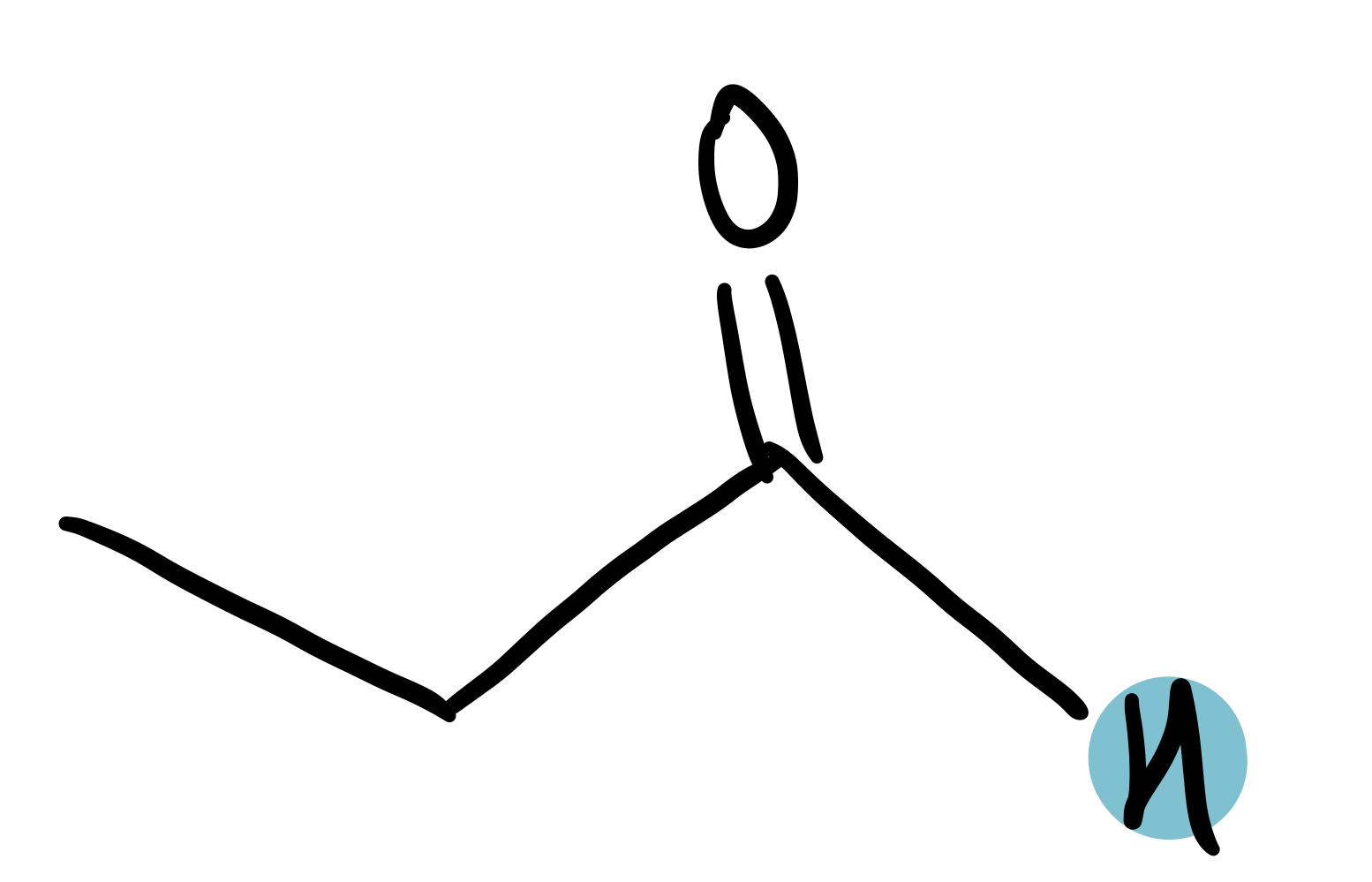
aldehyde
9.4-10.5
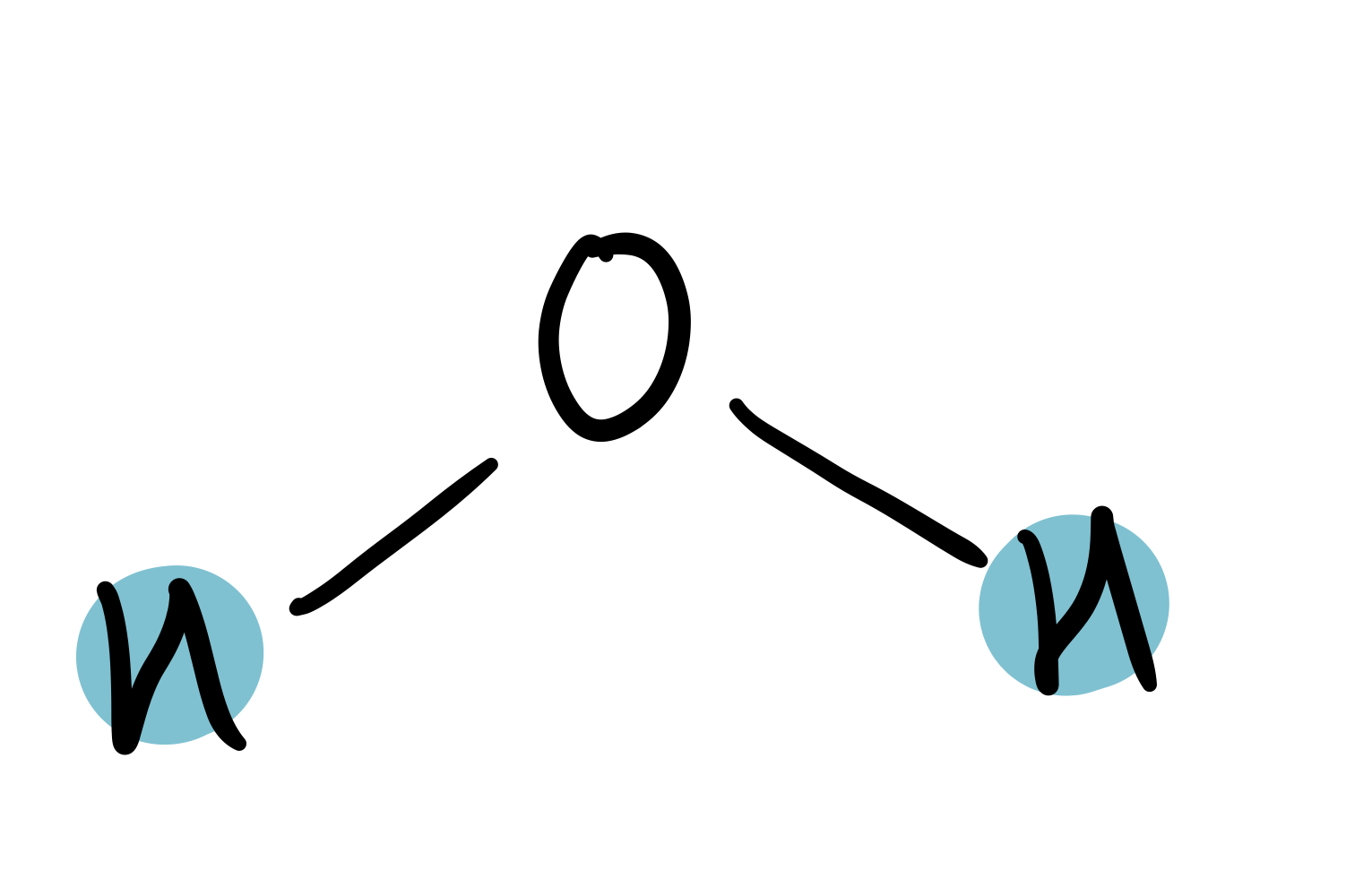
water
1.25-1.75
Ar-NH₂
3.1-6
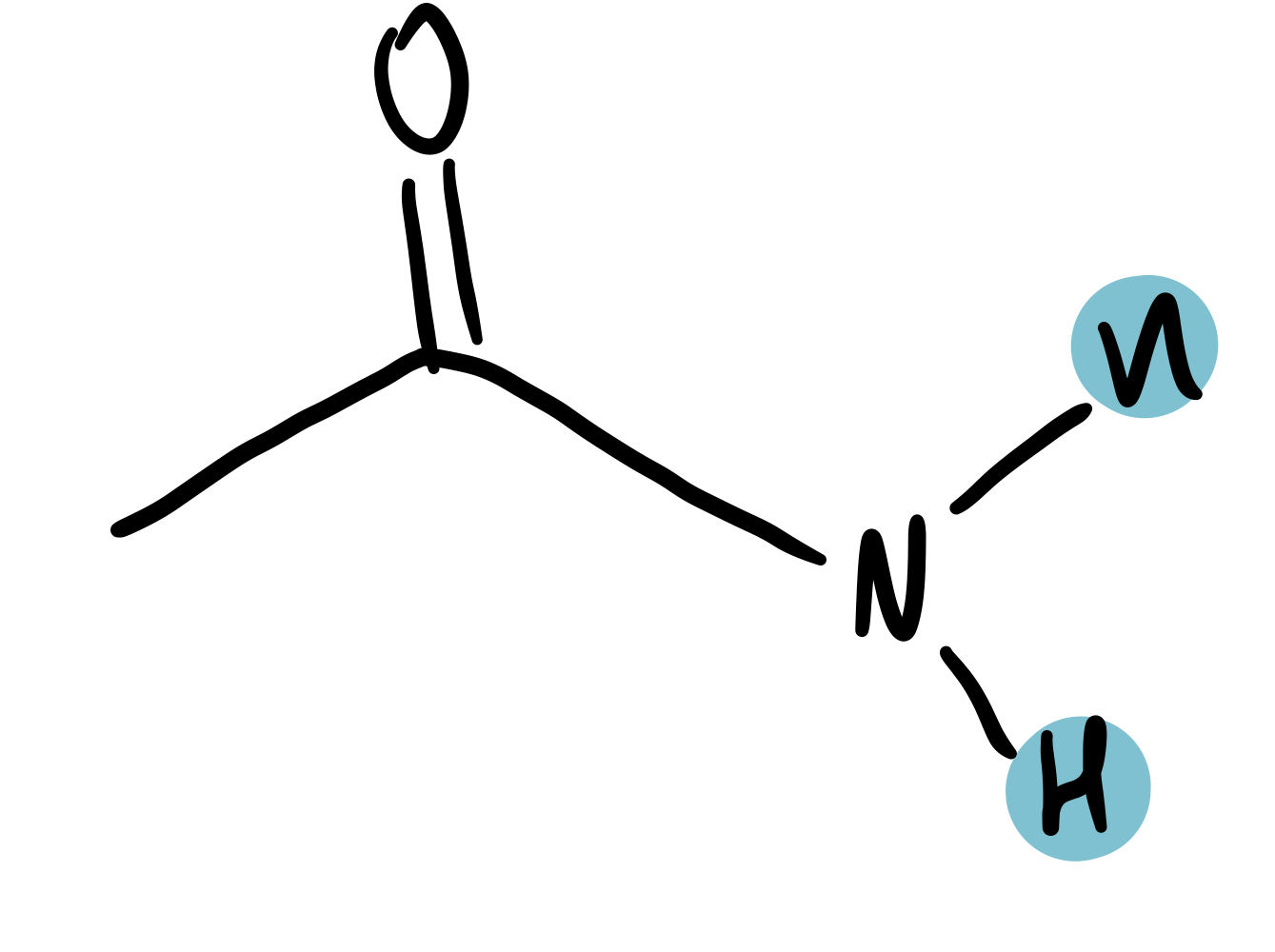
amide
5-12
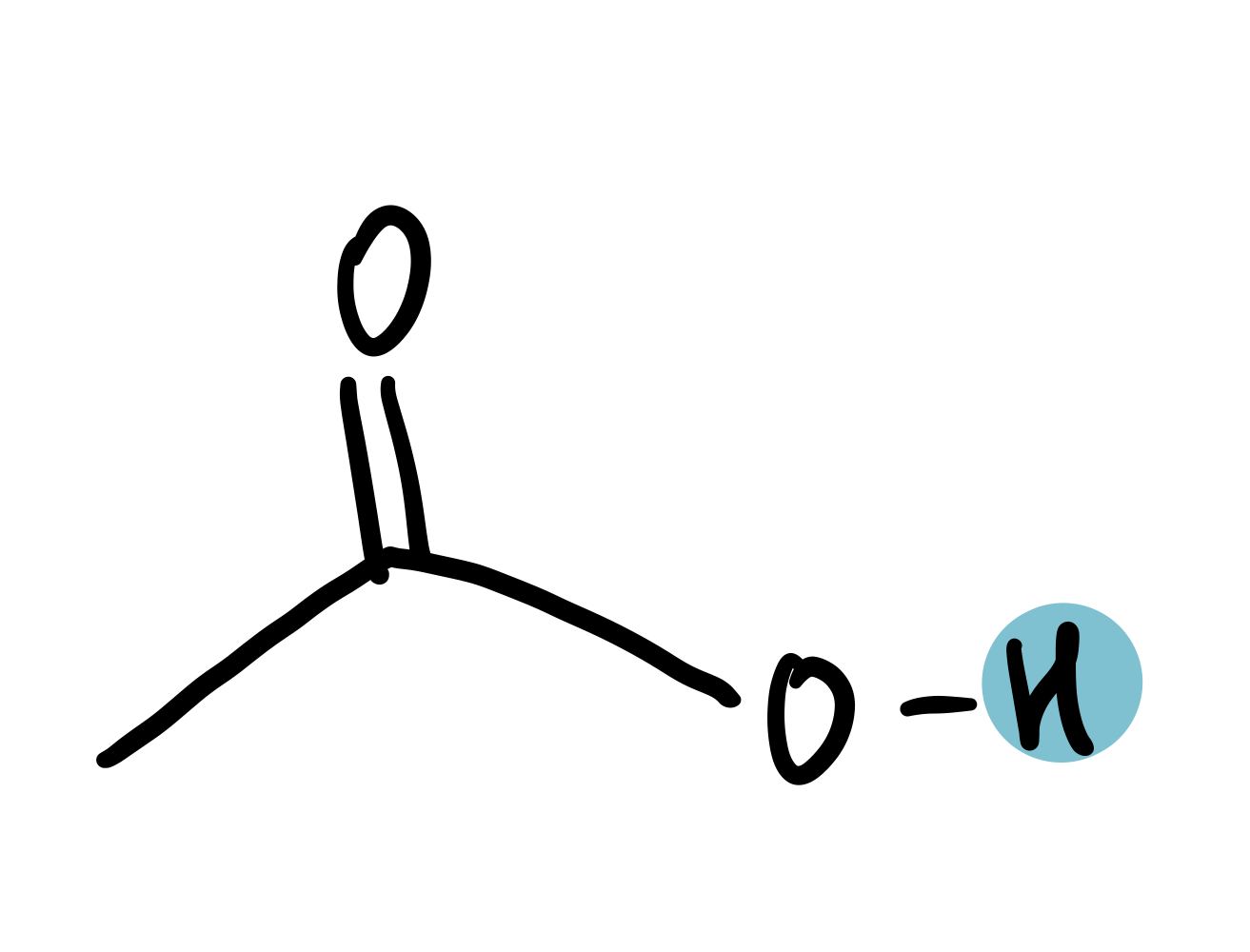
-COOH
9-12.5
What would you use a ‘D₂O shake’ on?
Molecules with N-H or O-H as the H will be replaced with D so their signals will disappear due to different spin states. But any acidic protons will be replaced in a molecule.
Why might we see no coupling in N-H and O-H containing molecules?
H bonding
exchangeable H’s rapidly swap in solution
protons they swap with are equally likely to be spin up or spin down
any nearby protons just see an average of the 2 spin states
Common splitting pattern for alkynes
triplet
reason for common alkyne splitting
long range coupling