chapter 6 keywords : electronegativity and the continuum of chemical bonding
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Electronegativity
Is the relative of attraction that an atom of a molecule has for the shared pair or pairs of electrons in a covalent bonds
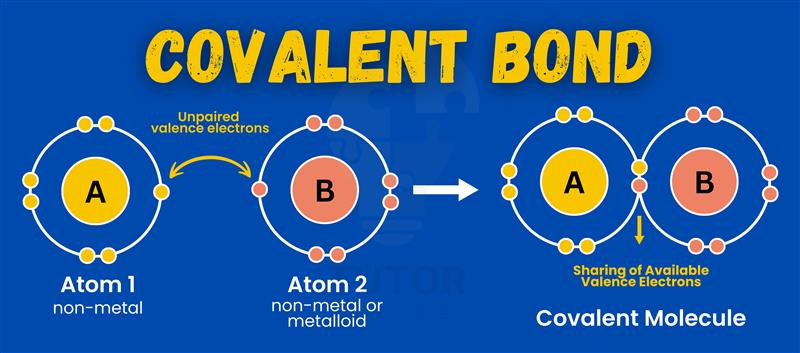
Polar covenant bond
Is a bond which there is unequal of the pair or pairs of electrons.
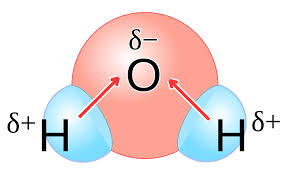
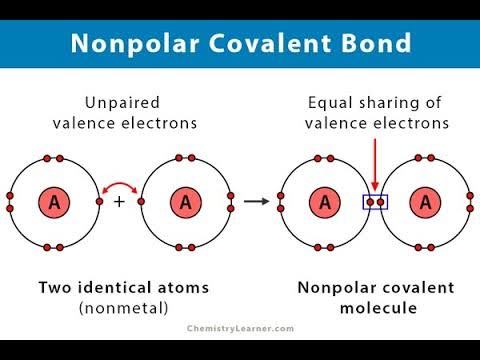
Pure covalent bonds
A bond which there is equal staring of the pair or pairs of electrons.
When do polar covalent bonds occur?
When the atoms bonded together have different electronegativity values
When do pure covalent bonds occur?
When the atoms bonded together have identical or very similar electronegativity values
Electronegativity difference for pure covalent bonding
0 - 0.4 inclusive
Electronegativity difference for a polar covalent
0.4 to 1.7 exclusive
What’s another name for pure polar covalent bonding
Non-polar covalent bonding
Electronegativity difference for covalent bonding
Less than 1.7 inclusive
Electronegativity difference for ionic bonding
More than 1.7
What is the range for the continuum of chemical bonding?
0 to 3.2
What role do Vanderwall forces play in molecules?
They play role roles in determining the boiling melting points and solubility

two conditions for a polar molecule
Bonds must be polar covalent
Molecule must be asymmetrical/lucky uniformity/bond don’t cancel out
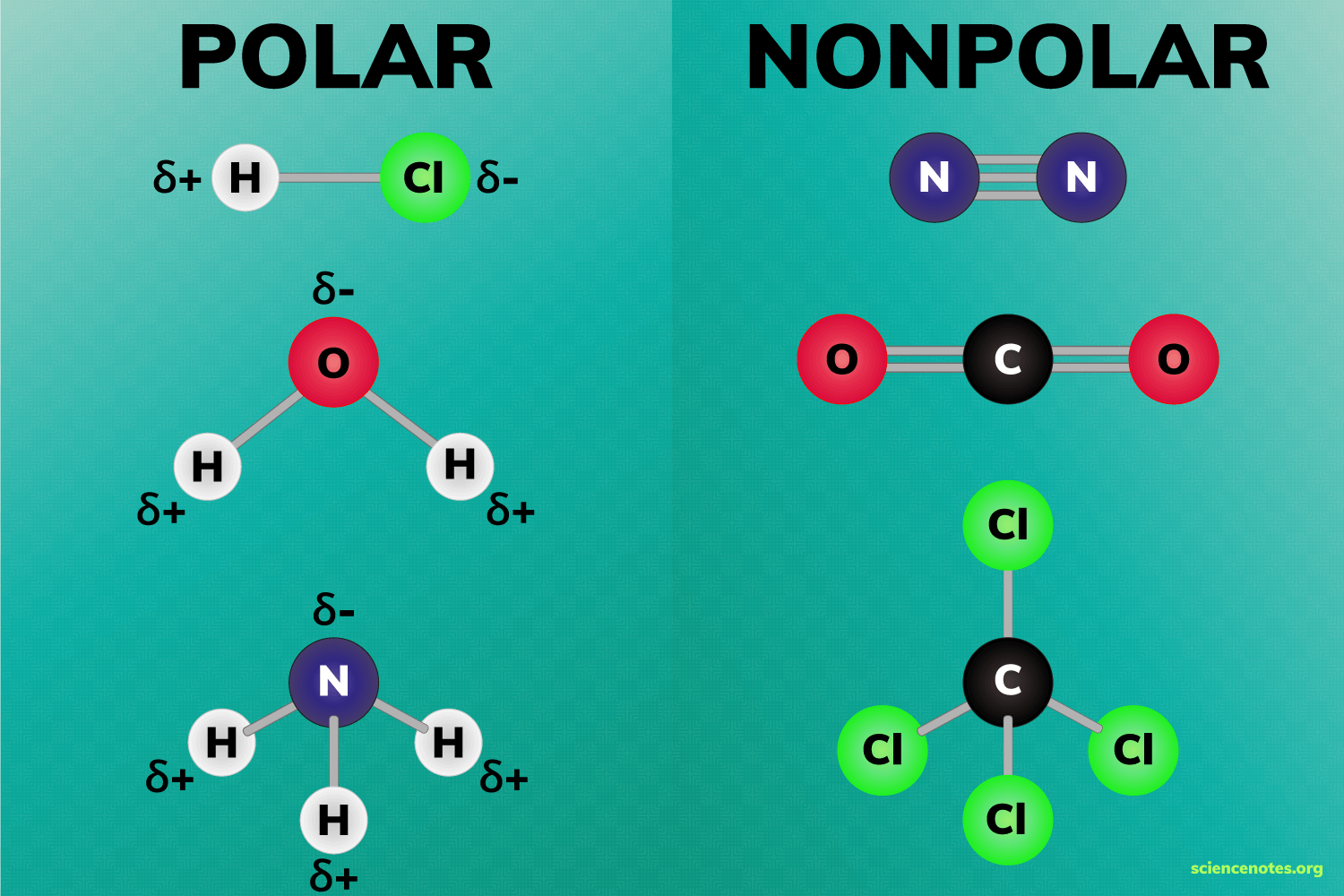
Example examples of polar molecules
H2O water
NH3 ammonia
Non-polar molecule
Molecule That has uneven distribution of electrical charges
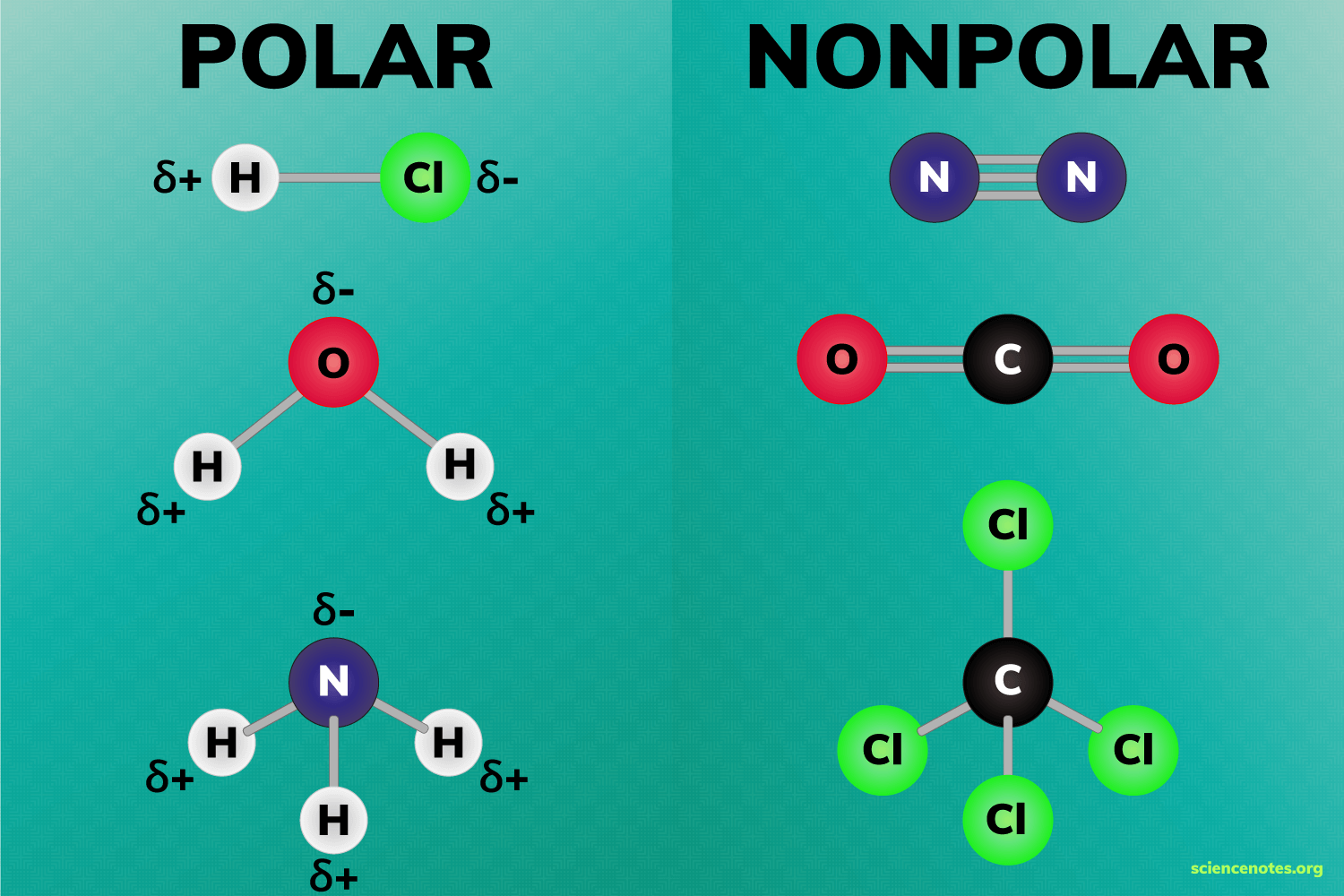
How do you know if a molecule is nonpolar?
It is symmetrical
Examples of nonpolar molecule shapes
Linear
Trigonal planar
Tetrahedral
Experiment to demonstrate whether a liquid is polar or non-polar
the effect of charged plastic rod on a stream of liquid
Polar liquid experiment
The liquid is attracted to the rod
The positive pills of water are attracted to the negative rod
Intramolecular bonding
Bonding that takes place within a molecule
examples of intramolecular bonding
Covalent bonding.
If there are no polar covalent bonds is the molecule polar or nonpolar
The molecule is nonpolar.
What elements are are very electronegative?
The very reactive non-metals
For a molecule to be polar what must be fulfilled?
Polar bonds
Asymettrical
What is the most electronegative element
Fluorine
What is intermolecular forces
The forces of attraction that exist between molecules
What is another name for intermolecular forces
Van Der Waals
What are van der waals forces
Weak , intermolecular forces of attraction that exist between all molecules.
Characteristic of van der Waal forces
Weaker than covalent bonds
Occur between ALL molecules
Strength decreases as distance increases
3 types of van der Waals
London dispersion forces
Permanent dipole-dipole forces inc hydrogen bonding
Ion-dipole forces
What role does van der waals Forces play
Boiling points
Melting points
Solubility