B1.1 carbohydrates and lipids
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
what are the four biochemical groups?
carbohydrates, lipids, proteins, and nucleic acids
how can carbon show the nature of covalent bonds?
all of the four molecules have carbon. carbon has 4 electrons on its outer ring, so two carbon atoms can share electrons with each other to create a covalent bond in which they share electrons to make full outer electron shells (octet rule)
how is carbon able to bond with so many other atoms?
carbon has 4 electrons on its outer ring, so it can make up to 4 single covalent bonds (for example with hydrogen) for it to be stable and have 8 electrons. it can also make double bonds (which are 4 covalent bonds) for example with oxygen, where it shares 4 with one and another 4 with another creating carbon dioxide.
what elements are common within molecules of living organisms?
oxygen, nitrogen, and phosphorus. they are found in carbohydrates, proteins, lipids, and nucleic acids, often forming covalent bonds with carbon and themselves.
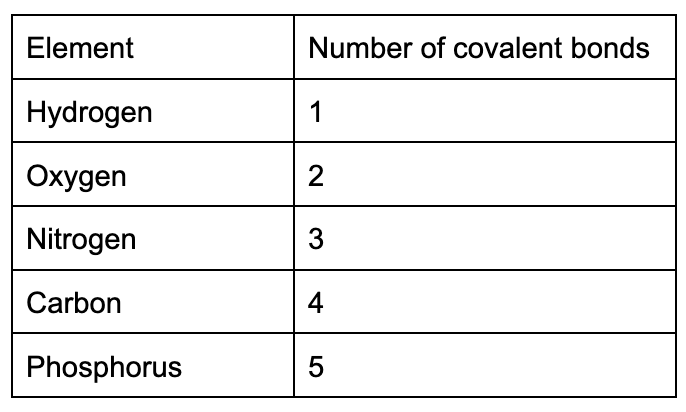
memorize this table the book says its important
what are the common categories and subcategories of molecules?
carbohydrates - mono/di/polysaccharides
proteins - no subcategory
lipids - triglycerides, phospholipids, steroids
nucleic acids - nucleotides (DNA, RNA, ATP)
what are macromolecules and the process of digesting them?
macromolecules are made up of smaller molecules (monomers). when you ingest food they are in the form of macromolecules, so digesting them takes chemical reactions called hydrolysis to break down the covalent bonds between the monomers. afterwards they are able to be absorbed in the bloodstream and circulated to body cells. after entering the cells, they are often built up into macromolecules again through condensation.
what are the monomers in the four main categories of molecules?
carbohydrates - monosaccharides
lipids - glycerol, fatty acids, phosphate groups
proteins (polypeptides) - amino acids
nucleic acids - nucleotides
what is an example of the process of hydrolysis and condensation? (for understanding)
eat taco with beef which is protein
hydrolysis reaction occurs resulting amino acids
amino acids are absorbed into the blood and taken to body cells
DNA in body cells directs specific condensation reactions to produce a specific protein from the amino acids
what is the process of condensation polymerization?
condensation bonds two amino acids with oxygen. the OH in the carboxylic-acid group of one bonds with the H int he amino group of the other amino acid, thus creating a byproduct of H2O. the new covalent bond created between the amino acids is called a peptide bond.
what is the process of hydrolysis?
hydrolysis is the exact opposite of condensation. it breaks the peptide bonds so the H2O is split into two components, for example a disaccharide into two monosaccharides, polysaccharides into many monosaccharides, and polypeptides (protein) to amino acids.
what are some examples of condensation reactions in the 4 macromolecule classes?
condensation can link multiple monomers to form polymers such as disaccharide and polysaccharides. it is also used in amino acids to form polypeptides and in nucleotide components to make DNA or RNA nucleotides (sugar phosphate backbone)
what is the difference between pentose and hexose monosaccharides?
it just means that they have 5 carbons for the backbone (pentose) or 6 carbons (hexose), which also makes the shape into a pentagon or hexagon in ring form. example is ribose for penta and glucose for hexa.
what is the importance of glucose and its properties?
glucose is a polar molecule because it has 5 of the alcohol (OH) functional group (the bond for O-H is polar covalent). its polarity allows it to have properties such as molecular stability due to strong covalent bonds, high solubility in water, ease with transport because of solubility in water, and its ability to yield lots of chemically energy when covalent bonds are broken which makes it a good energy store.
how is starch an example of polysaccharides as energy storage compounds?
starch is a polysaccharide made of hundreds of glucose monomers. for the starch to be very compact, the plant uses two kinds of bonds between glucose molecules : alpha 1-4 linkage and alpha 1-6 linkage. in one starch called amylose, coiling occurs as glucose are bonded by only 1-4 linkages, so the molecule is linear but in a helix shape. in another starch, amylopectin, branching occurs where the linear molecule branches off using the 1-6 linkage.
what are other properties of starch that make it an energy storage compound?
a molecule of starch is very large so it has low solubility in water, which helps a plant easily store starch. even though the molecule is large its also compact, so when a plant is photosynthesizing and producing lots of glucose, it can add more glucose molecules to amylose or amylopectin by condensation reactions. alternatively, when a plant needs to reserve its glucose, hydrolysis reactions can break them away from the starch
how is glycogen an example of polysaccharides as energy storage compounds?
glucose is also made of glucose monomers that are bonded in similar patterns as amylopectin, however with more branching. animals and humans store excess glucose as glycogen, so their reserves are kept within our liver and muscle tissue. an advantage of storing glycogen is that they’re not readily soluble in the cytoplasm and other fluids, so they don’t affect the osmotic balance in living tissues unlike individual glucose molecules.
how is cellulose a structural polysaccharide?
starch and glycogen use the alpha form of glucose, while cellulose uses the beta form. in cellulose the 1-4 carbon linkages between the glucose molecules through condensation requires every second molecule to be upside down, so it can be oriented by the hydroxyl group of carbon 1 with the hydroxyl group of carbon 4. the result is a very linear polymer with no branches, continuing as bundles of fibres to form larger bundles held together by cross linking attractions of hydrogen bonds.
what is the importance of cellulose as a structural polysaccharide in its function as a cell wall?
the reason why cellulose has so many compact, tight, and strong bonds is to act as a structural molecule in nature (cell wall). cellulose is also insoluble in water, so the fibres allow water and other substances to pass freely into and out of plant cells. its not considered as an energy storage molecule because few organisms produce the enzyme necessary to digest it.
what are conjugated molecules?
when two or three of the categories of molecules are bonded together to accomplish a specific function. for example, carbohydrate + protein = glycoprotein
how do glycoproteins work in cell-cell recognition?
many membrane proteins are glycoproteins. they are responsible for cell signalling, cell to cell adhesion, transportation of molecules, recognition of body cells vs non body cells, etc
how do glycoproteins work in ABO blood types?
glycoproteins are found on the surface of red blood cells to determine a persons ABO blood type. there are two possible glycoproteins, A and B called antigens, because their presence can trigger the immune system aka the ability of white blood cells to recognize self from non-self (diseases).
what specific blood types have antigens?
AB have both A and B antigens, so their immune system will not be triggered by the presence of either. type O don’t have either of them so their immune system will be triggered by the presence of A and B antigens. people who only have A or B is triggered by the presence of the one they don’t have. this is why type O’s are universal donors and why AB are universal recipients.
what can lipids be categorised into?
fats, oils, waxes, steroids
what are the hydrophobic properties of lipids?
lipids dissolve well in non-polar solvents but not in water. organisms evolved to take advantage of this limited solubility, for example to conjugate (join/link) the lipid with another molecule like glycolipids and lipoproteins
why do lipids have hydrophobic properties?
the molecules contain many areas of hydrocarbons, so only hydrogen and carbon. the covalent between them is a non-polar covalent bond.
what are triglycerides?
lipids that contain one glycerol molecule and three fatty acid molecules, formed from condensation reactions and resulting in 1 triglyceride with 3 water molecules.
what are phospholipids?
molecules that are formed if an inorganic phosphate group replaces one of the three fatty acids. so it would be 1 glycerol, 2 fatty acid, an inorganic phosphate and the result would be 1 phospholipid and 3 molecules.
what are saturated fatty acids?
fatty acids that contain single bonds between carbons. all other carbon bonds are with hydrogen (except for the carboxyl group), so the molecule is “saturated” with hydrogens. they have high melting point and are solid at room temperature. triglycerides containing only saturated fatty acids are called fats. many animals store energy in this form.
what are monounsaturated fatty acids?
fatty acids that have one double bond between two of the carbons in the hydrocarbon chain, but the location of the double bond can vary. triglycerides containing one or more of them have a lower melting point than saturated fatty acids, and are liquid (oil) at room temperature. animals and plants store energy in this form
what are polyunsaturated fatty acids?
fatty acids that have more than one double bond in the hydrocarbon chain. the number and location of the double bond varies. triglycerides with them have a low melting point and are liquid at room temperature. many plants store energy in this form.
what is adipose tissue?
tissue composed of cells that store fat in the form of triglycerides. the quantity of triglycerides stored depends on the organism’s calorie intake vs calories burned.
how is adipose tissue an example of triglycerides being important for energy storage?
condensation reactions form triglycerides and are most common when an organism eats food that has more calories than they are using. hence, they can be used to supply energy when sufficient foods are not available for metabolic needs. they’re useful for long-term energy storage because they’re insoluble in body fluids and won’t move from the adipose storage sites. they also provide 2x as much energy released by carbohydrates
how are triglycerides used as thermal insulators for body temp and habitat?
a thick layer of adipose tissue is typical for animals that live in cold regions. birds and mammals are endotherms, which means they maintain a steady internal temp regardless of the environment. for example, seals, walruses, and whales are all endotherms because their adipose tissue is the blubber and is found between the skin and muscles. it helps trap the heat made by their inner metabolic activities.
what are amphipathic molecules?
molecules that have both hydrophilic and hydrophobic regions.
how are phospholipids amphipathic molecules?
a phospholipid has a polar head and a non polar tail. they create a bilayer to solve the problem of having hydrophobic tails, as they extend towards each other to keep away from aqueous solutions inside and outside the cell. the polar phosphate groups are attracted to the aqueous solutions so they arrange themselves on the outside of the bilayer. this the foundation of the plasma membrane.
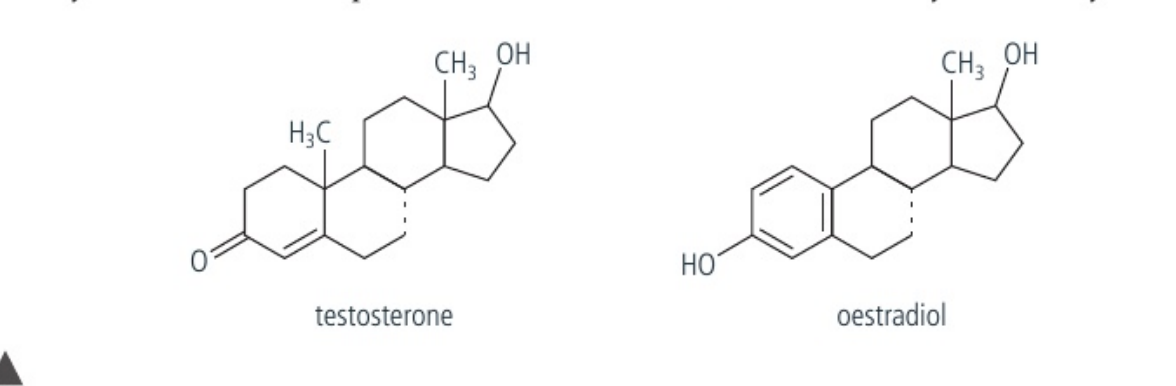
what non-polar steroids can pass through the phospholipid bilayer? (memorize the structure)