Oxidation Reactions
1/54
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
55 Terms
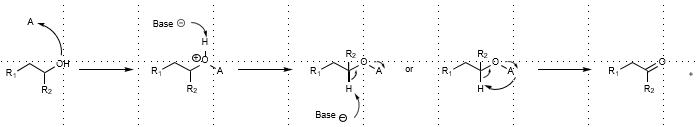
General Mechanism of Alcohol Oxidation
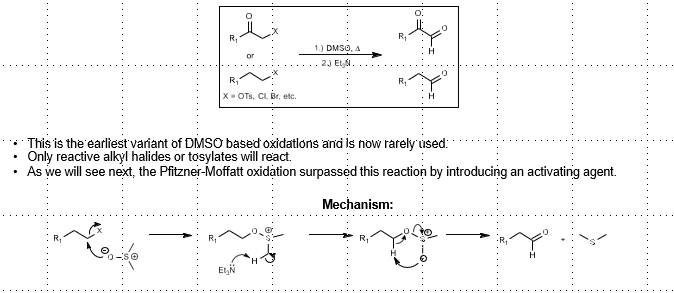
Kornblum Oxidation: (Sulfur-based alcohol oxidation)
Alkyl halide oxidation (or alpha halo-carbonyl)
Only reactive alkyl halides or tosylates will react. 1) DMSO, heat 2) Et3N/Base
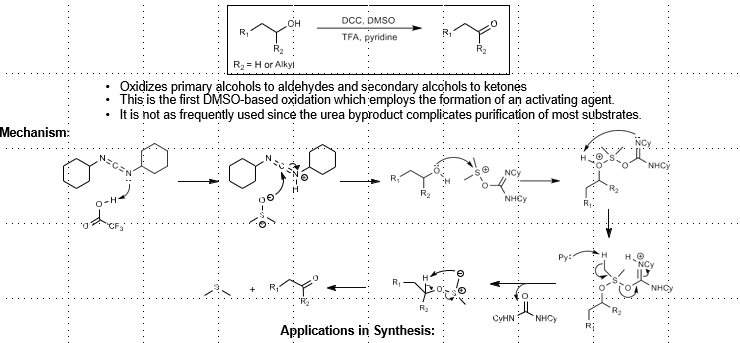
Pritzner-Moffatt Oxidation (Sulfur-based alcohol oxidation)
Alcohol Oxidation
DCC, DMSO, TFA
DCC activation by TFA, attack by DMSO
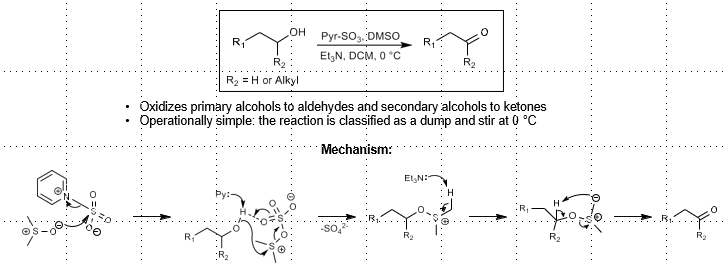
Parikh-Doering Oxidation (Sulfur-based alcohol oxidation)
Alcohol oxidation
Pyr-SO3, DMSO, Et3N
Dump and stir
Corey-Kim Oxidation: (Sulfur-based alcohol oxidation)
Alcohol Oxidation
N-chlorosuccinamide, DMS, Et3N
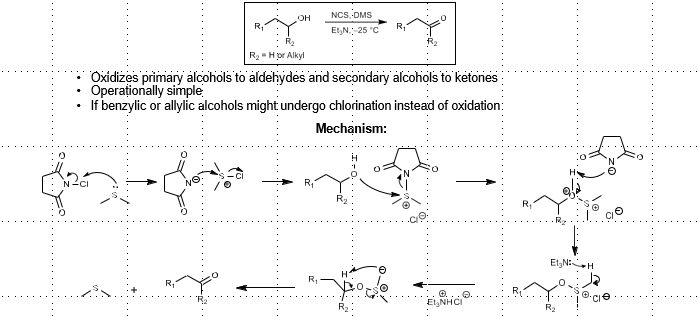
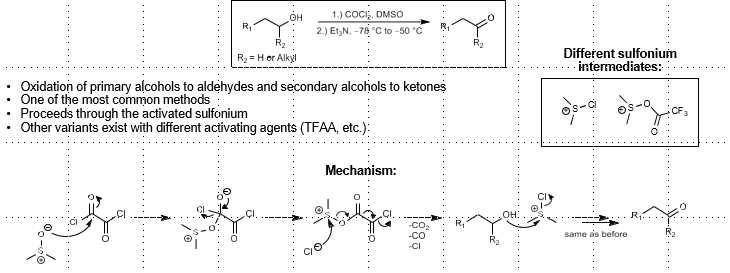
Swern Oxidation: (Sulfur-based alcohol oxidation)
Alcohol oxidation
COCl2, DMSO
Et3N
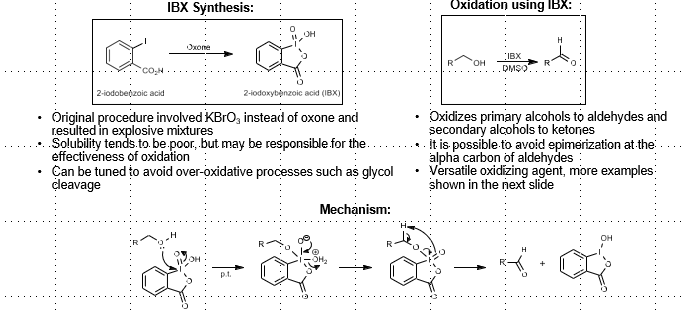
2-iodoxybenzoic acid (IBX) Oxidation
Alcohol oxidation
IBX
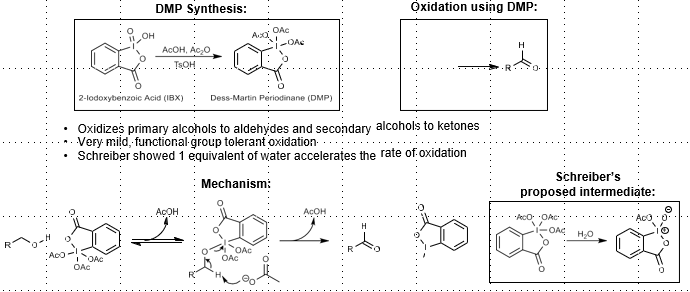
Dess-Martin Periodinane
Alcohol oxidation
DMP
N-oxoammonium Mediated Oxidation
Alcohol to ketone/aldehyde
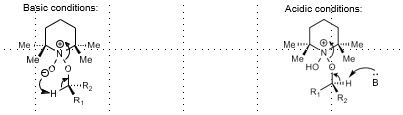
Ley Oxidation
Alcohol oxidation
Ley oxidation - TPAP, NMO
tetrapropylammonium perruthenate, NMO
in DCM gives aldehyde/ketone,
with H2O gives carboxylic acid
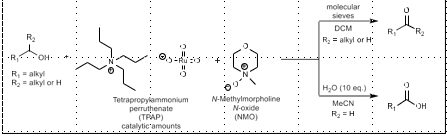
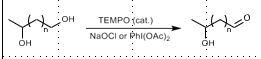
TEMPO Oxidation
PRIMARY alcohol to ketone/aldehyde
TEMPO, NaOCl or PhI(OAc)2
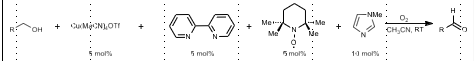
Stahl Oxidation
PRIMARY alcohol to ketone/aldehyde
Cu(I) salt, bpy, TEMPO, imidazole, O2
ABNO Oxidation: Alcohol to Amide
PRIMARY alcohol oxidation, directly to amide
Cu(I), ABNO
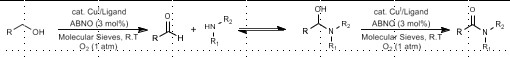
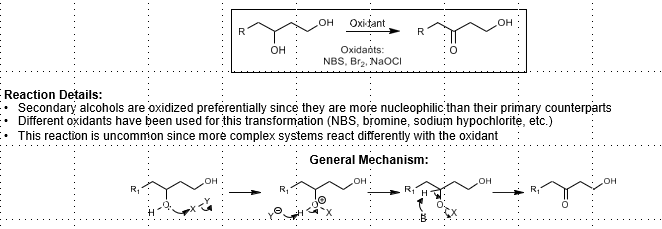
Chemoselective Secondary Alcohol Oxidation
Oxidants: NBS, Br2, NaOCl
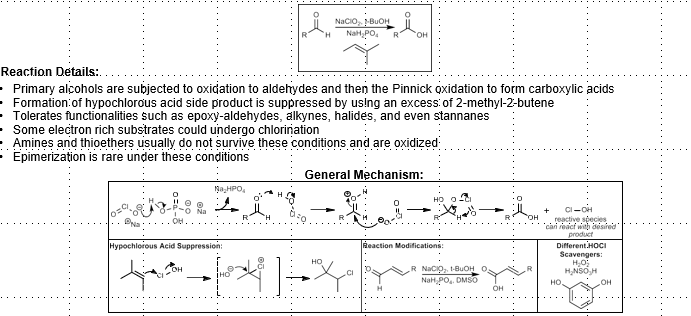
Pinnick Oxidation
Aldehyde to carboxylic acid
NaClO2, t-BuOH, NaH2PO4
2-Me-2-Butene to suppress hypochlorous acid
Chlorite adds to aldehyde, deprotonates in elimination
Chromium Reagents - alcohol oxidation
Alcohol to carboxylic acid: Jones (CrO3, H2SO4, H2O —→ H2CrO4)
Alcohol to Ketone/aldehyde: Collins (CrO3-pyr2, DCM)
Alcohol to Ketone/aldehyde: Corey (PCC, pyridinium chlorochromate) - slightly acidic
Alcohol to Ketone/aldehyde: PDC (Cr2O7, solvent). Milder PCC. Can be secondary selective based on solvent
Allylic Oxidation with Chromium reagents: Oxidative Transposition
PCC or PDC. Via [3,3] sigmatropic rearrangement
![<p>PCC or PDC. Via [3,3] sigmatropic rearrangement</p>](https://knowt-user-attachments.s3.amazonaws.com/78bdc00f-46d0-494a-9bbc-0fd312a20284.png)
Allylic Oxidation: Oxidative Transposition
Enone from allylic alcohol
3 mechanistic paths: Dissociative, [1,3]-rearrangement, SN2
Use of N-oxoammonium salt
![<p><strong>Enone from allylic alcohol</strong></p><p>3 mechanistic paths: Dissociative, [1,3]-rearrangement, SN2</p><p>Use of N-oxoammonium salt</p>](https://knowt-user-attachments.s3.amazonaws.com/0a16083a-91ff-4af6-8fc0-72fb9e724fd3.png)
Allylic Oxidation using Chromium reagents
Allylic carbonylation
PCC, PDC, or CrO3 (3-5)-DMP
Mechanistic speculation: Radical pathway
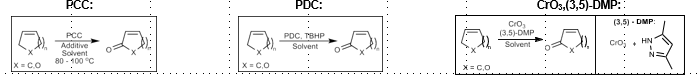
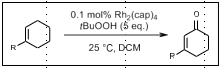
Doyle Oxidation: Allylic carbonylation
Allylic carbonylation
Rh2(cap)4, tBuOOH
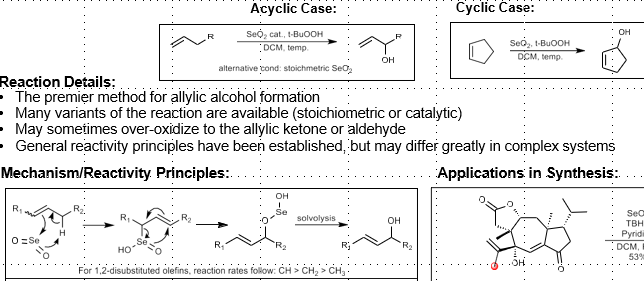
Selenium Dioxide Oxidation: Allylic Hydroxylation
Allylic hydroxylation
Selectivity: Tri substituted olefins react at more substituted end of double bond.
Order of reactivity: CH2>CH3>CH
Terminal olefins prefer endocyclic vs exocyclic (i.e inside the cycle versus outside)
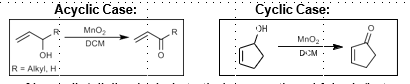
Manganese Dioxide Oxidation
Oxidation of allylic alcohol
Corey Modification: Allylic alcohol to methyl ester (NaCN, cat. AcOH, MeOH). Cyanohydrin intermediate, oxidation to carbonyl cyanide, then attack by methanol to form methyl ester)
Can also cleave diols
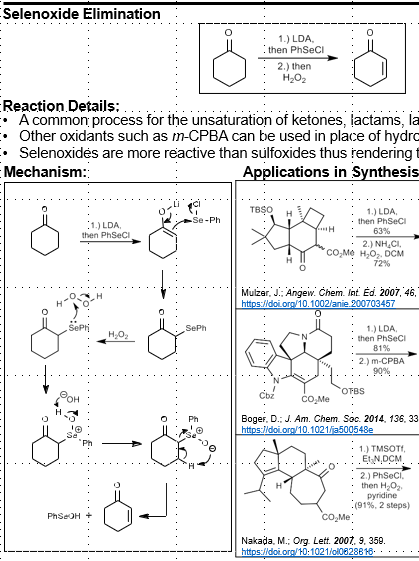
Selenoxide Elimination: Unsaturation of Carbonyl
Unsaturation of carbonyl compounds
LDA, then PhSeCl
Then, H2O2
PhSeCl reagent. Can use other peroxides instead of H2O2
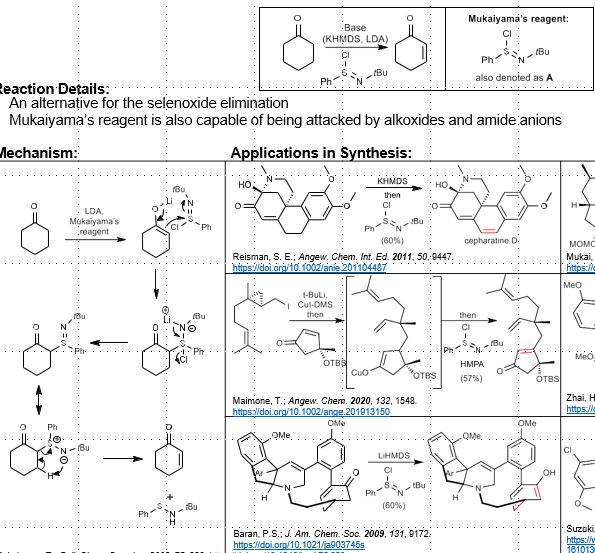
Mukaiyama’s reagent: Unsaturation of Carbonyl
Unsaturation of carbonyl compounds
Base, Mukaiyama’s reagent
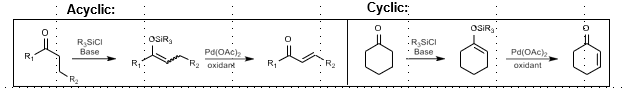
Saegusa-Ito Oxidation: Unsaturation of carbonyl compounds
Unsaturation of carbonyl compounds
Silane + base, then Pd(OAc)2 + Oxidant
Via silyl enol ether intermediate
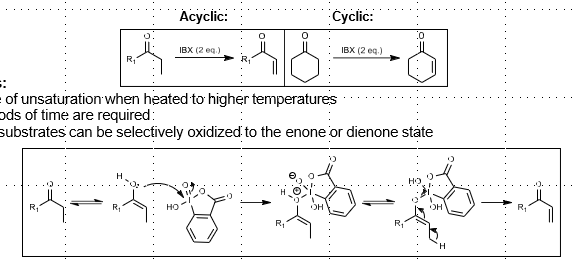
IBX Unsaturation
Ketone to alpha-beta unsaturated ketone
Halogen Elimination: Unsaturation of carbonyl
Unsaturation of carbonyl compounds
CuX2, NCS, or X2
Mechanism: Bromide formation, elimination
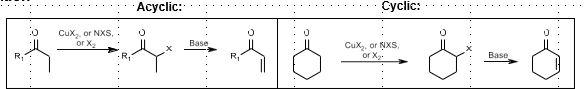
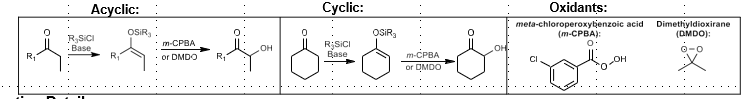
Rubottom Oxidation: Alpha hydroxylation of carbonyl
Alpha hydroxylation of carbonyl compounds
Silane, then mCPBA
Mechanism:
Via silyl enol ether intermediate. Peroxide attack forms epoxide, which forms a ketone + alpha silyl ether intermediate via rearrangement. Protonation yields product.
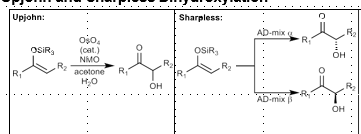
Upjohn and Sharpless Hydroxylation: Alpha hydroxylation of carbonyl compounds
Nonselective (Upjohn) or stereoselective (Sharpless) Alpha hydroxylation of carbonyl compounds
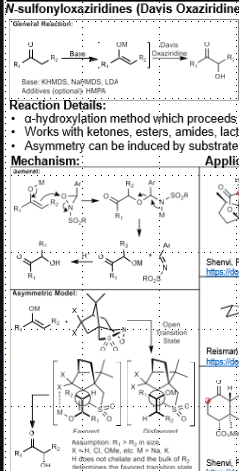
Davis Oxaziridine: Alpha hydroxylation of carbonyl compounds
Alpha hydroxylation of carbonyl compounds
Base, then Davis oxaziradine
Enantioselective variant possible
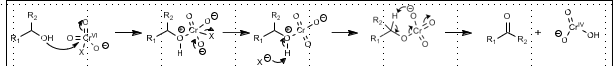
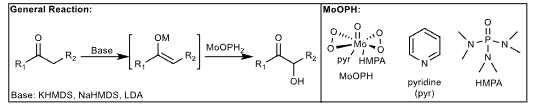
MoOPH Oxidation: Alpha hydroxylation of carbonyl
Alpha hydroxylation of carbonyl compounds
Mechanism: Via Metal-enol ether adduct. Oxygen is from MoOPH.
Modifications:
From nitrile compound: Ketone formed
From Sulfone: Ketone formed
From alkene: enantioselective hydroxylation from borane intermediate (from hydroboration)
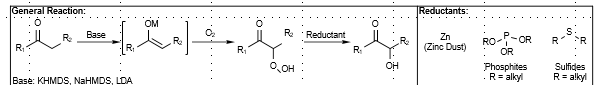
Alpha Hydroxylation with Oxygen
Alpha hydroxylation of carbonyl compounds
Reductant required
Mechanism: Via Metal-enol ether adduct. Oxygen is from MoOPH. Peroxide intermediate via radical recombination (triplet oxygen). Reductant cleaves hydroperoxo intermediate.
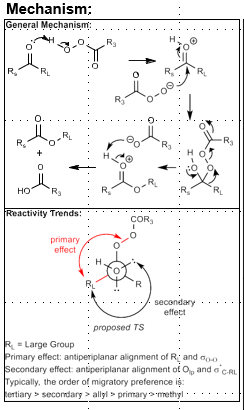
Baeyer-Villiger Oxidation
Ester synthesis
tBuOOH
Large group (RL) needs to be anti periplanar to O-O bond.
More subsittuted group migrates (tertiary>secondary>allyl>primary>methyl)
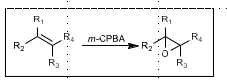
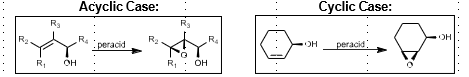
Prilezhaev Epoxidation
Nucleophillic epoxidation
“Butterfly mechanism”
More acidic peroxyacid = faster reaction
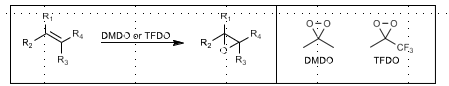
DMDO or TFDO Epoxidation
TFDO can also do C-H bond hydroxylation
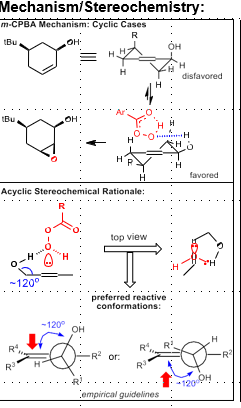
Henbest Epoxidation
Required allylic alcohol
Stereoselective, directed by H-bonding between alcohol and peracid
Henbest Epoxidation Mechanism/TS
120 degree dihedral angle between alcohol directing group and alkene (minimize A1,3 interactions)
In cyclic case, half-chair formation dictates disfavored vs favored face (by whether half chair is stable or not)
Sharpless Directed Epoxidation
Allylic alcohol required
Stereoselective, directed by chelation.
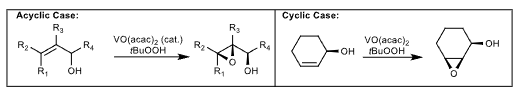
Sharpless Epoxidation Mechanism/TS
50 degree angle between alcohol and alkene, determines which face is most favorable (minimize A1,3 interactions). Top face or bottom face favored
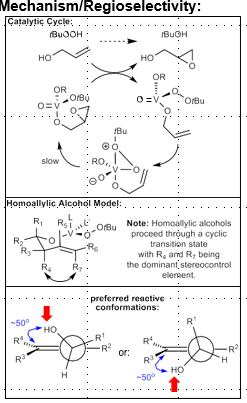
Sharpless Asymmetric Epoxidation
Allylic alcohol required
Ti(OiPr)4, tBuOOH, Diethyltartrate (+ or -)
Alcohol in bottom right corner, (+)-DET attacks from top face
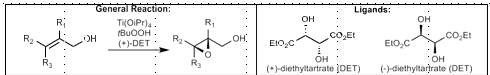
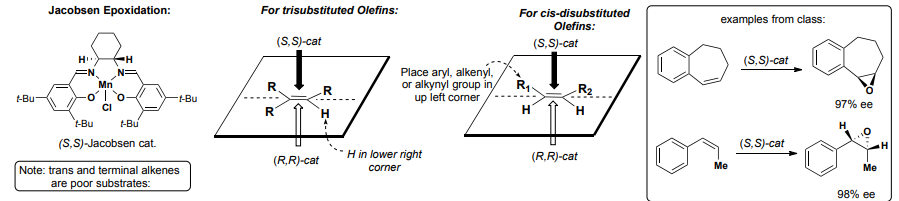
Jacobsen Epoxidation
Best for conjugated systems (alpha-beta unsaturated ketones, styrenes, etc.)
Mn catalyst, NaOCl (bleach) oxidant
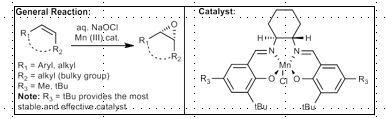
Shi Epoxidation
Best for non-conjugated, non-allylic alcohols
Oxone, Fructose, Potassium carbonate base
Nucleophilic Epoxidation
Activated alkene required (EWG)
Hydroxide, peroxide, water
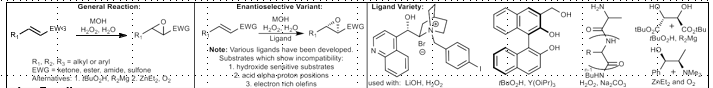
Upjohn Dihydroxylation
Syn dihydroxylation.
OsO4, NMO
In cyclic case, dependent on current ring conformation/substituents
If NaIO4 after, Lemieux-Johnson Oxidation (Oxidative cleavage of diols)
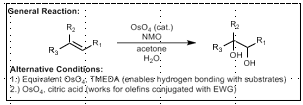
Upjohn Dihydroxylation Mechanism/TS
120 degree dihedral angle required that minimized A1,3 interaction. However, OsO4 approached from OPPOSITE face of preferred conformer.
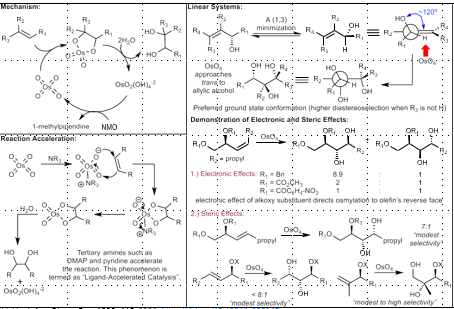
Sharpless Asymmetric Dihydroxylation
AD Mix: K2CHO3, K3[Fe(CN)6], K2OsO4-H2O
![<p>AD Mix: K2CHO3, K3[Fe(CN)6], K2OsO4-H2O</p>](https://knowt-user-attachments.s3.amazonaws.com/7a050ad1-5a61-4e12-8fe1-d9dff787b94a.png)
Jacobson Epoxidation Stereochemistry
((S,S) TOP, (R,R) BOTTOM) for BOTH cases—>
For tri-sub olefins: H in lower right corner
For Cis-sub olefins: Aryl, alkenyl, or alkynyl group in top left corner
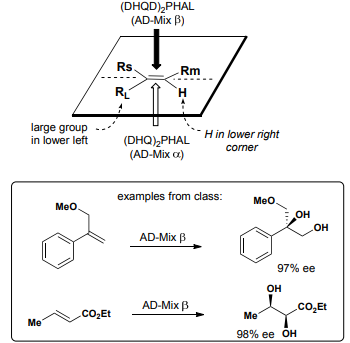
Sharpless Asymmetric Dihydroxylation Stereochemistry
H in lower right corner, Large group in lower left.
Alpha bottom, Beta top
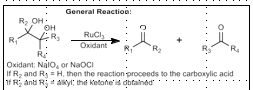
Ruthenium Trichloride Oxidative Cleavage
Stronger oxidative cleavage. Will oxidize aldehydes to carboxylic acids
Alpha hydroxy ketones/aldehydes are also cleaved
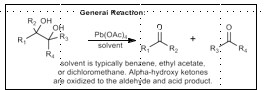
Lead Tetraacetate Oxidative Cleavage
Oxidative cleavage of diols
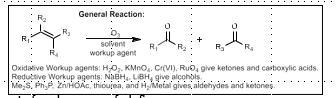
Ozonolysis
Oxidative cleavage of Olefins
O3
Lemieux-Johnson Oxidative Cleavage
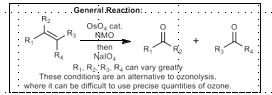
Ozonolysis-Schreiber’s Modifications
O3, MeOH, p-tsOH
then NaHCO3, DMS: Acetal + ketone/aldehyde
then Ac2O, Et3N: Acetal + ester
O3, MeOH, NaHCO3
then Ac2O, Et3N: Aldehyde + ester
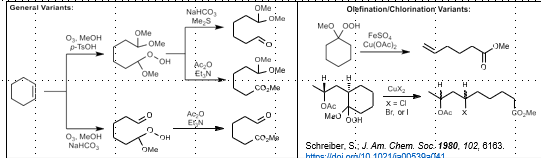
Schenk Ene Photooxygenation
Allylic alcohol from alkene
O2, photosensitizer, then reductant
Dehydration step gives alkene (need alpha proton available in one of the alkene substiuents)
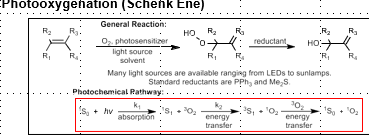
Oxidation of Olefins via Singlet Oxygen
[4+2] cycloaddition, reductant forms 1,4- allylic diol
[2+2] cycloaddition, reductant forms 1,2 diol
Kornblum-DeLaMare gives a gamma-hydroxy alpha-beta unsaturated ketone
Kornblum-DeLaMare + Furan gives alpha-beta unsaturated hydroxy lactone
![<p><strong>[4+2] cycloaddition, reductant forms 1,4- allylic diol</strong></p><p><strong>[2+2] cycloaddition, reductant forms 1,2 diol</strong></p><p></p><p>Kornblum-DeLaMare gives a gamma-hydroxy alpha-beta unsaturated ketone</p><p>Kornblum-DeLaMare + Furan gives alpha-beta unsaturated hydroxy lactone</p>](https://knowt-user-attachments.s3.amazonaws.com/a4fe1b7e-0f44-4e2b-8469-4848ded49663.png)