lec 2 (mcbride) - lipids, carbs, part 1: basic structures
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
monosarccharides
simplest carbohydrates
carbohydrates = carbon-based molecules high in OH groups
empirical formula = (CH2O)n
can have additional groups or modifications
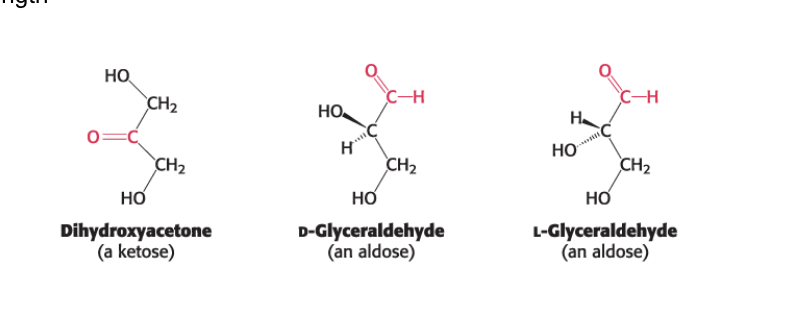
better described as polyhydroxy aldehydes and ketones (and their derivatives)
monosaccharides are aldehydes or ketones that contain 2 or more OH groups
smallest monosaccharides composed of 3 carbons
monosaccharides exist in many isomeric forms
also known as “simple sugars”
monosaccharides = carbohydrates that are 3-7 carbons in length
monosaccharide nomenclature
nomenclature based on carbon-chain length
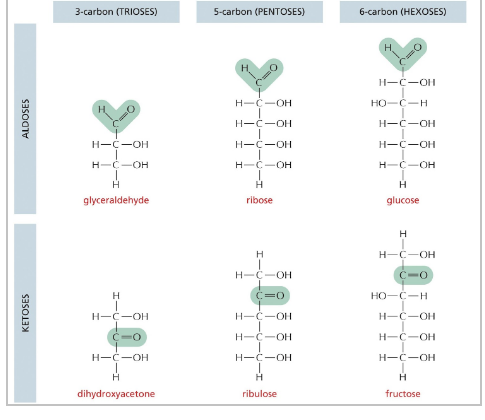
3 Cs = trioses
4 Cs = tetroses
5 Cs = pentoses
6 Cs = hexoses
7 C = heptoses
nomenclature also based on the identity of the most oxidized group
keto group = ketose
aldehyde group = aldose
sugars commonly found in cells
glyceraldehyde
ribose
glucose
dihydroxyacetone
ribulose
fructose
monosaccharides exist in a variety of isomeric forms
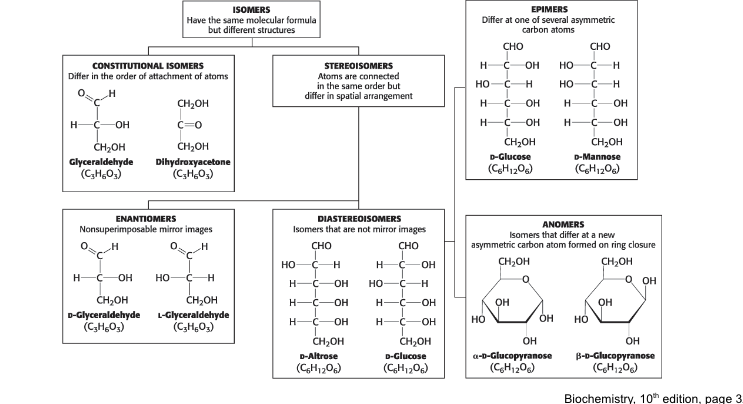
constitutional isomers
molecules with identical molecular formulas that differ in how the atoms are ordered
stereoisomers
molecules that differ in spatial arrangement but NOT bonding order
have either D or L configuration (most monosaccharides from vertebrates have D config)
can be enantiomers (mirror images of each other) or diastereoisomers (NOT mirror images of each other)
enantiomers → all Cs have altered chirality
diastereoisomers → if only some Cs have altered chirality
# possible = 2n where n = # of asymmetric C atoms
common monosaccharides
have multiple asymmetric carbon atoms
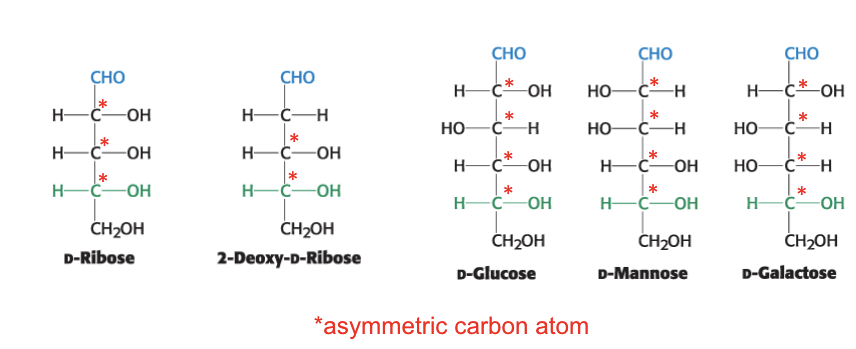
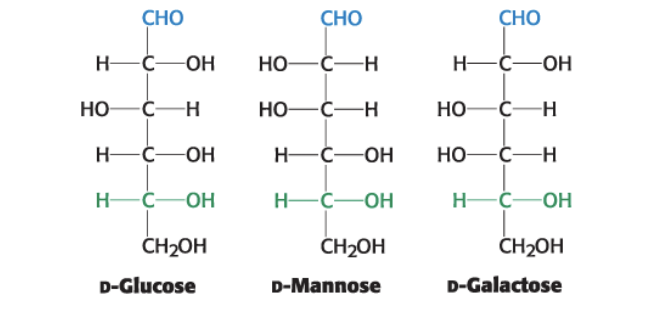
epimers
they are diastereoisomers differing in configuration only at a single asymmetric center
glucose + mannose = epimers; C2 has altered chirality
glucose + galactose = epimers; C4 altered
mannose + galactose = diastereoisomers b/c altered chirality at 2 AND 4
overview of isomeric forms of monosaccharides
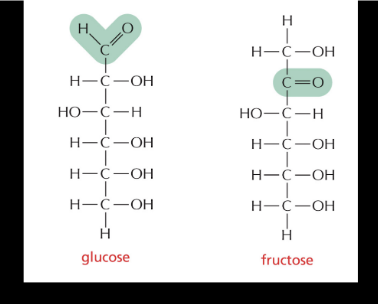
glucose and fructose are what type of isomers
constitutional
same molecular formula but DIFFERENT connectivity
monosaccharides and rxns
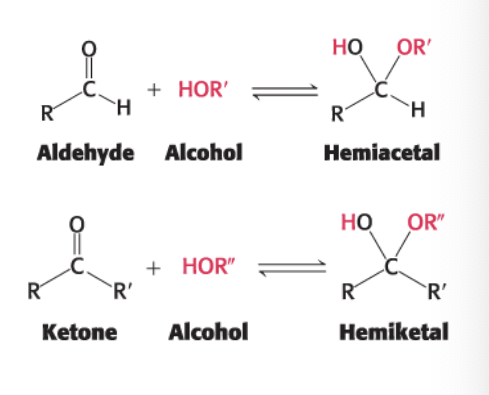
each end of a monosaccharide can react together
aldehyde can react with alcohol to form hemiacetal
ketone can react with alcohol to form hemiketal
monosaccharides are predominately in…
ring formation inside cells (in solution)
in aqueous soln, aldehyde or ketone group of a sugar molecule tend to react with OH of the same molecule → closing molecule into ring
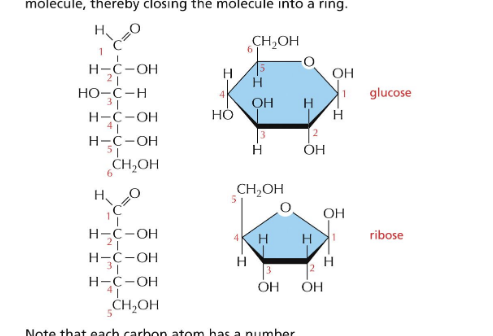
glucose forms a __ ring
pyranose
called pyranose b/c of similarity to pyran
anomers = isomers that differ at a new assymmetric carbon formed on ring closure (alpha & beta)
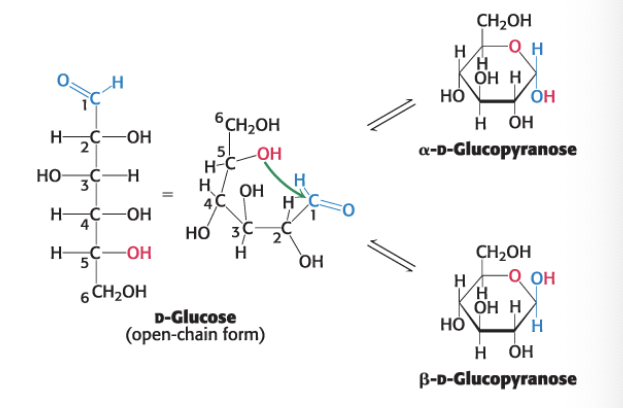
fructose forms a __ ring
furanose
called furanose b/c of similarity to furan
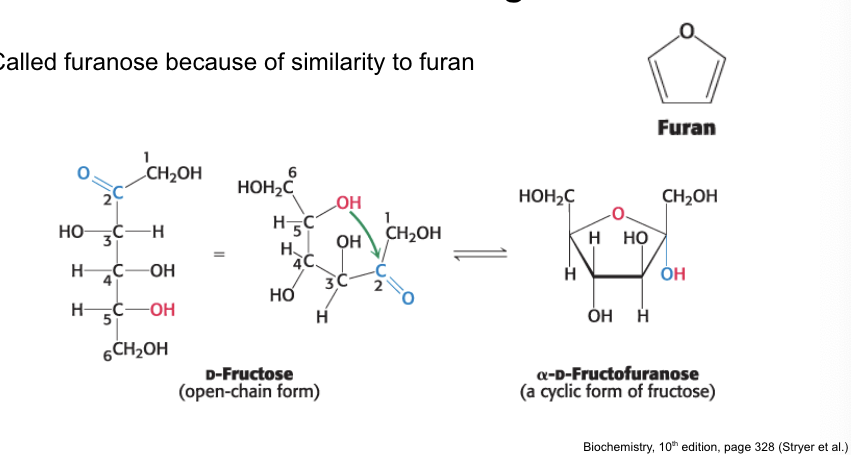
most common monosaccharides exist primarily…
in their ring forms
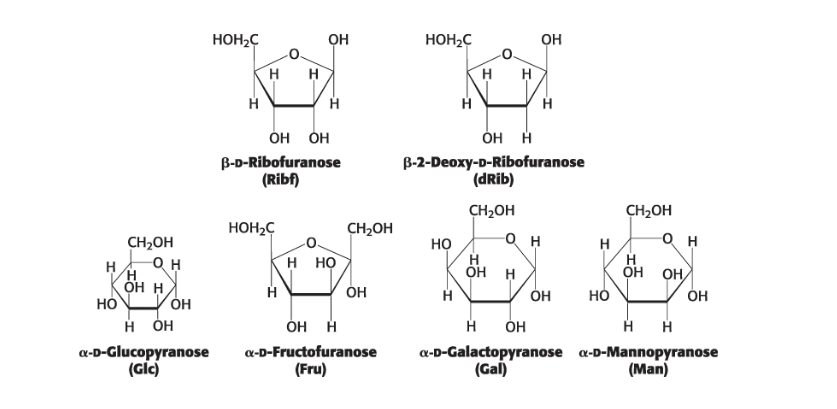
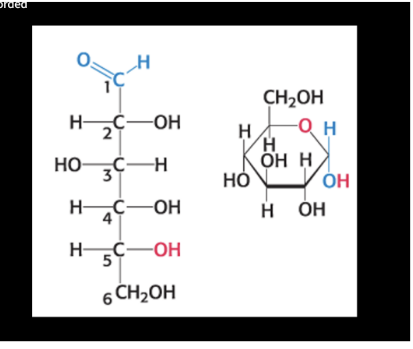
how many asymmetric carbons?
open chain = 4 (everything but end Cs)
ring = 5
polysaccharides or complex carbohydrates are ___
multiple monosaccharides
oligosaccharides = sugars that contain 2 or more monosaccharides linked by O-glycosidic bonds
disaccharides (such as sucrose) consist of 2 sugars
identity of glycosidic bond is determined by…
carbons linking the monosaccharides
“reducing sugars” have anomeric forms due to possessing a reducing end
reducing end = has a free anomeric C that can form the open chain form
nonreducing end = hsa an anomeric C in a glycosidic linkage that CANNOT convert to open chain form
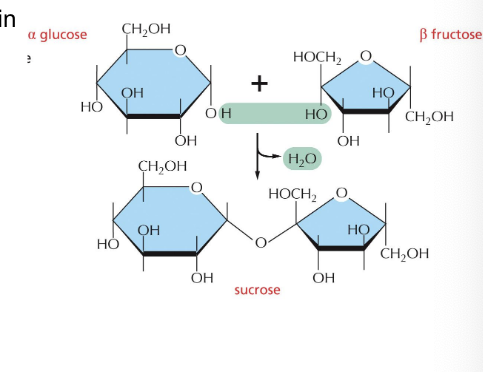
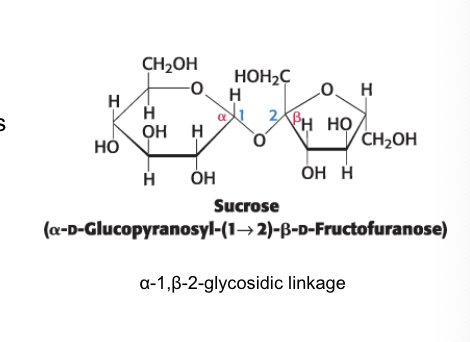
sucrose
formed from glucose and fructose
sucrose = disaccharide of sugar cane or sugar beets that consists of glucose linked to fructose
the anomeric C of glucose is linked to the anomeric C of fructose
configuration is α for glucose and β for fructose
not a reducing sugar
can be cleaved by sucrase (invertase)
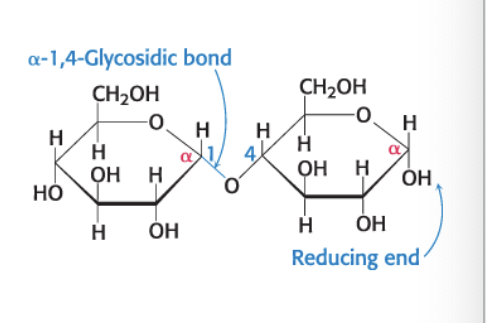
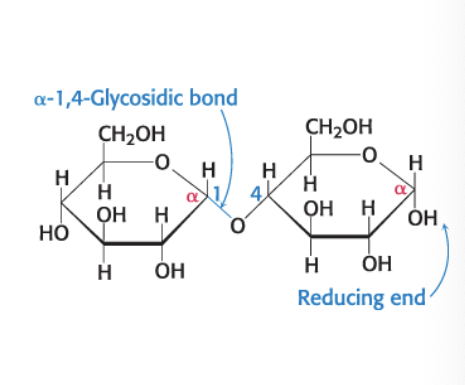
maltose
disaccharide of 2 glucose molecules
α-1,4-glycosidic linkage = glycosidic linkage between the α-anomeric form of C-1 on one sugar and the OH oxygen atom on C-4 of the adjacent sugar
maltose = disaccharide resulting from the hydrolysis of large oligosaccharides that consist of 2 linked glucose molecules
joined by α-1,4-glycosidic linkage
can be hydrolyzed to glucose by maltase (α-glucosidase)
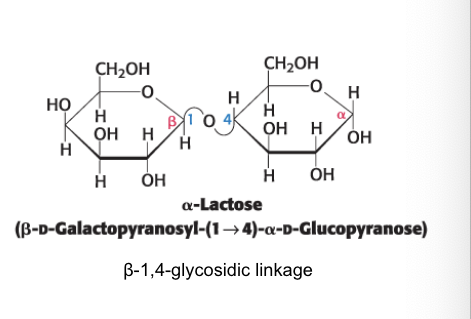
lactose
formed from galactose and glucose
lactose = disaccharide of milk that consists of a galactose linked to glucose
can by hydrolyzed by lactase in humans beings and β-galactosidase in bacteria
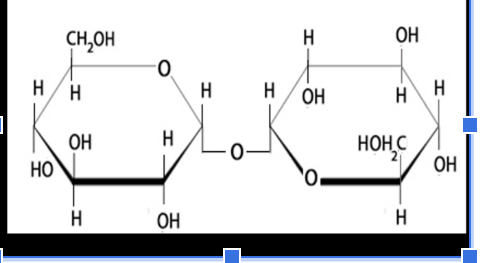
what is the glycosidic linkage
α,1,1-glycosidic bond (the C that is connected to OH and NOT methyl)
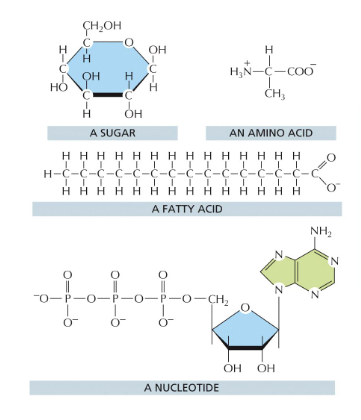
what is used to assemble larger macromolecules
monomeric building blocks or subunits
sugars → polysaccharides, glycogen, and starch (in plants)
fatty acids → fats and membrane lipids
amino acids → proteins
nucleotides → nucleic acids
each polymer is formed from small molecules (called monomers) linked together by covalent bonds
glucose is stored as…
macromolecule glycogen to provide energy in times of need
free glucose CANNOT be stored b/c high concentrations will disturb the cell’s osmotic balance
polysaccharides (glycans) = large polymeric oligosaccharides formed by the linkage of multiple monosaccharides
plays role in energy storage and structural integrity
homopolymer = polymer in which all the monosaccharide units are the same
glycogen
is a large, branched polymer of glucose residues
most common homopolymer in animal cells
storage form of glucose
most glucose units are linked by α-1,4-glycosidic linkages
hydrolyzed by α-amylase
branching increases the surface area to allow better access for enzymes to rapidly breakdown glycogen
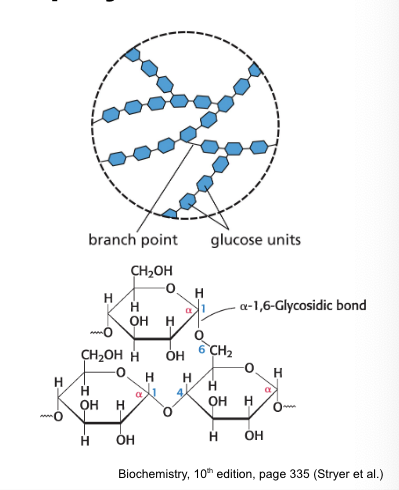
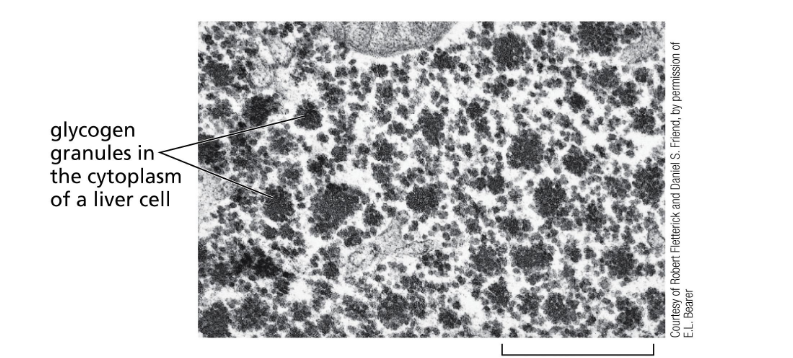
glycogen is stored in the…
liver
glycogen granules in the cytoplasm of liver cell
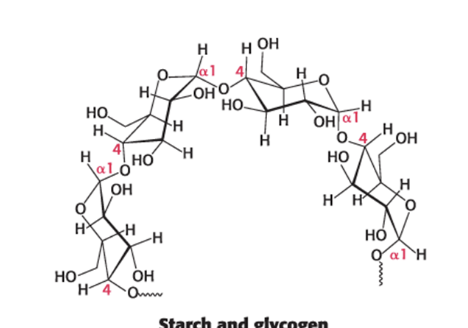
glycosidic linkages in mammals
determine polysaccharide structure: glucose storage in mammals
α-1,4 linkages favor bent, helical structures → more suitable for storage
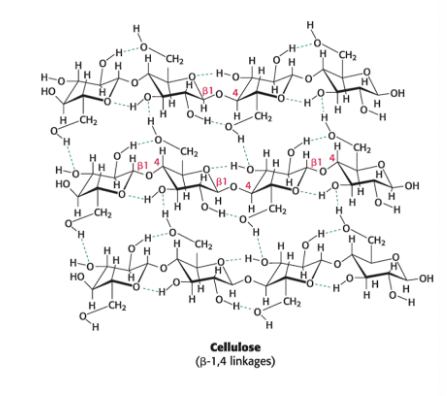
glycosidic linkages in plants
β-1,4 linkage favor straight chains → optimal for structural purposes
glycogen and energy production
glycogen provides glucose for energy production
when cells need more ATP than they can generate from food molecules taken in from the bloodstream → they break down glycogen
glycogen = shorter-term storage of energy
glycogen has the largest stores in liver and muscle
during fasting, liver cells release glucose derived from breakdown of their glycogen stores → bloodstream for use by other tissues while muscle cells hoard their supplies for their own use
an avg adult humans stores enough glycogen for only about a day of normal activities (energy demands) but enough fat to last for nearly a month
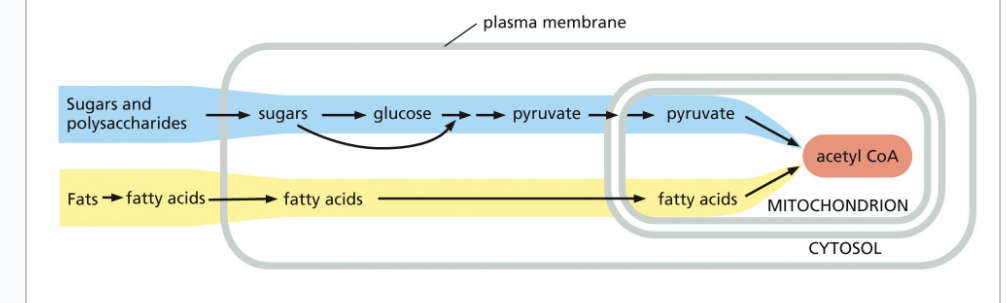
glycogen phosphorylase
is an enzyme which breaks down glycogen when cells need more glucose
fatty acids
have 4 major physiological roles
fatty acids are fuel molecules
fatty acids are building blocks of phospholipids and glycolipids
many proteins are modified by the covalent attachment of fatty acids which functions to target proteins to membrane locations
fatty acids derivatives serve as hormones and intracellular messengers
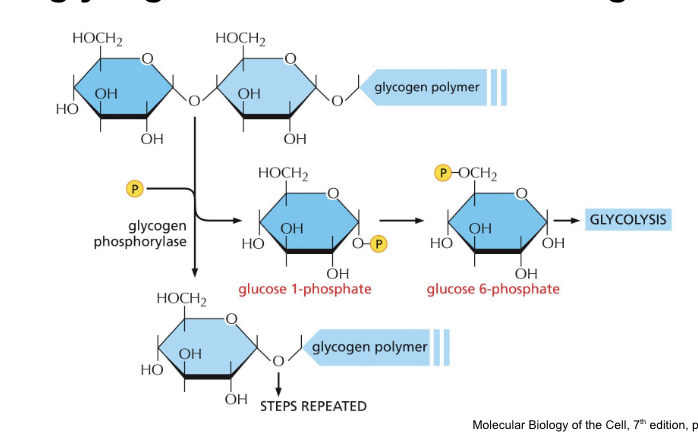
fatty acid structure
determined by double bonds in hydrocarbon tail
hundreds of different kinds of fatty acids exist
some have one or more double bonds in their hydrocarbon tail = unsaturated
double bond = rigid and creates a kink in the chain; rest of the chain is free to rotate around the other C-C bonds
NO double bonds = saturated
all fatty acids have a carboxyl group (COOH) at one end and a large hydrocarbon tail at the other
fatty acids are the building blocks for…
lipids
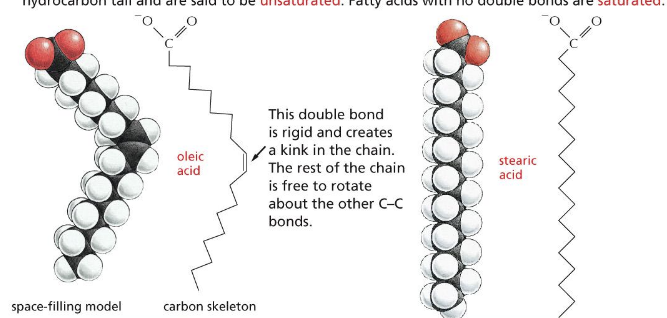
triacylglycerols (TAGs)
triacylglycerols (TAGs) are the storage form of fatty acids as uncharged esters with glycerol
fatty acids are stored in cells as an energy reserve (fats and oils) through an ester linkage to glycerol to form triaglycerols also known as triglycerides
how to identify the chemical composition of the fatty acids within the lipid molecule
first # = # of Cs
second # = # of double bond
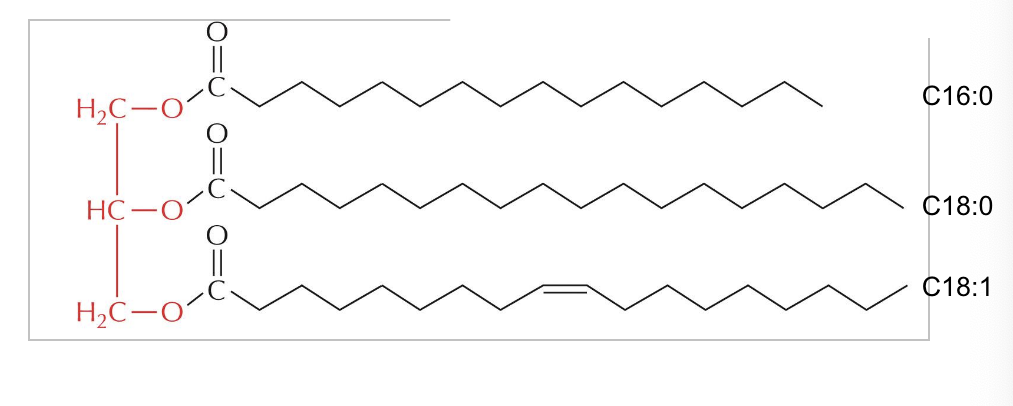
triglyceride structure
determined by fatty acid composition
3 fatty acid chains are linked to this glycerol thru ester bonds
simple triglyceride = when the 3 fatty acid tails are identical
mixed triglyceride = when the fatty acid tails are different
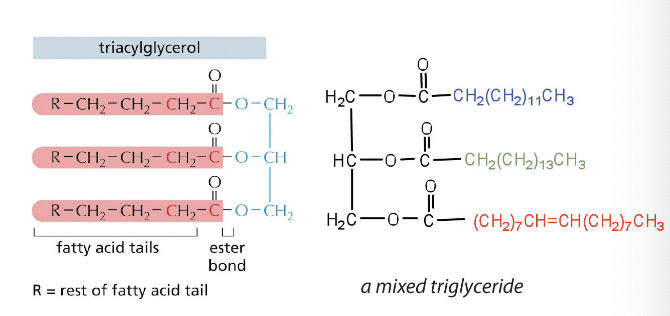
lipids
lipids with saturated fatty acids have higher energy density than unsaturated fatty acids
straight chains can be packed together very tightly → allowing them to store energy in a compact form
lipids with saturated fatty acids = solid at room temp (fats)
lipids with unsaturated fatty acids = liquid at room temp (oils)
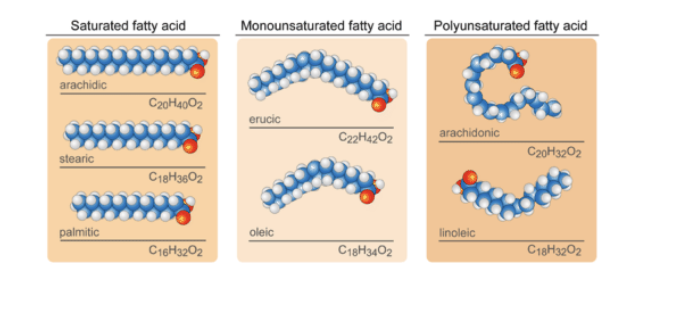
TAGs and energy stores
TAGs are highly concentrated energy stores
triacylglycerols (neutral fats, triglycerides) = uncharged esters of fatty acids with glycerol
stored mainly in adipose tissue
stored also by muscle for energy needs
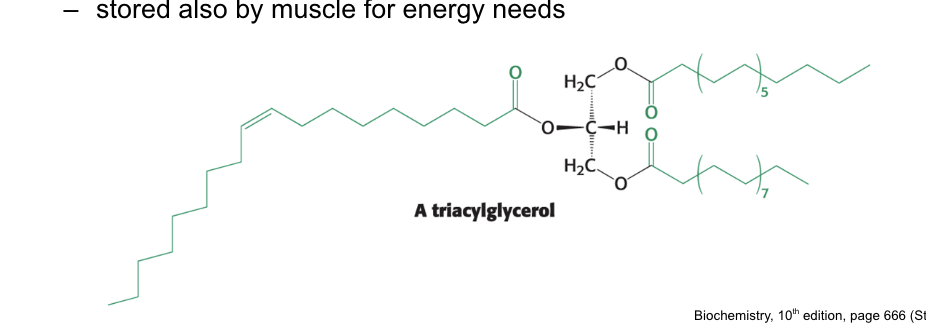
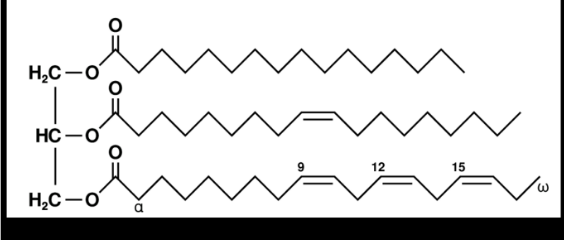
what are the identities of the fatty acids in this lipid?
TG(16:0,18:1,18:3)
TAGs are physically stored in…
adipose tissue
adipose tissue = fuel-rich, white tissue that is located throughout the body, notably under the skin (subcutaneous fat) and surrounding internal organs (visceral fat)
adipocytes = fat cells that make up adipose tissue
major site of triacylglycerol accumulation
specialized for triacylglycerol synthesis, storage and mobilization into fuel
TAGs form…
lipid droplets
lipid droplets = large globules formed by the coalescence of triacylglycerols
may occupy most of the adipocyte volume
surrounded by a phospholipid monolayer and proteins required for lipid metabolism
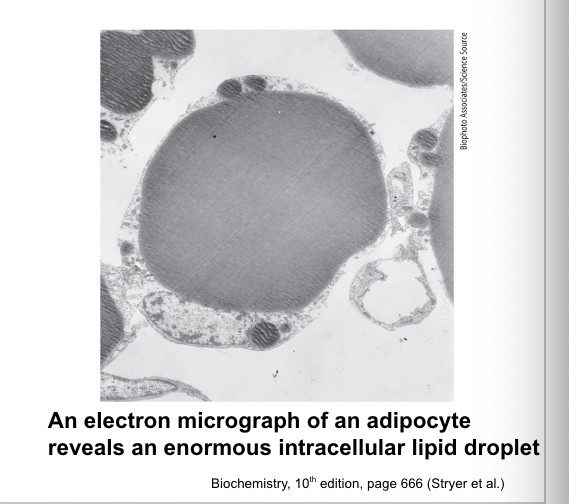
TAG degradation…
produces free fatty acids
lipases = intestinal enzymes that degrade triacylglyceroles to FFAs and monoacylglycerol
secreted by pancreas
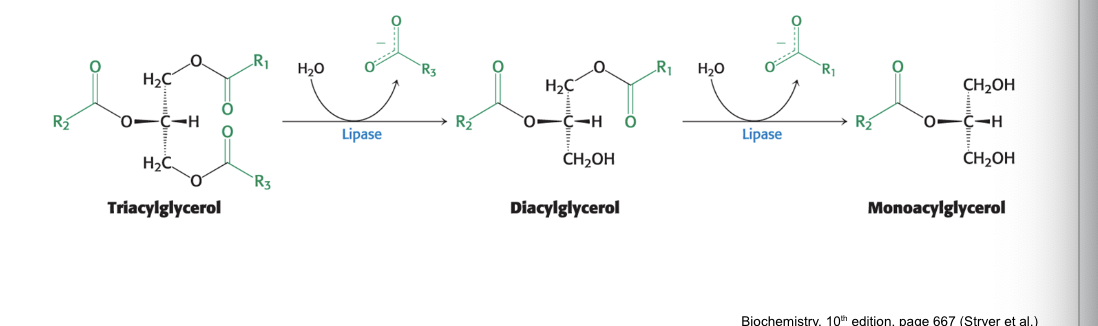
FFAs used as…
fuel by many tissues
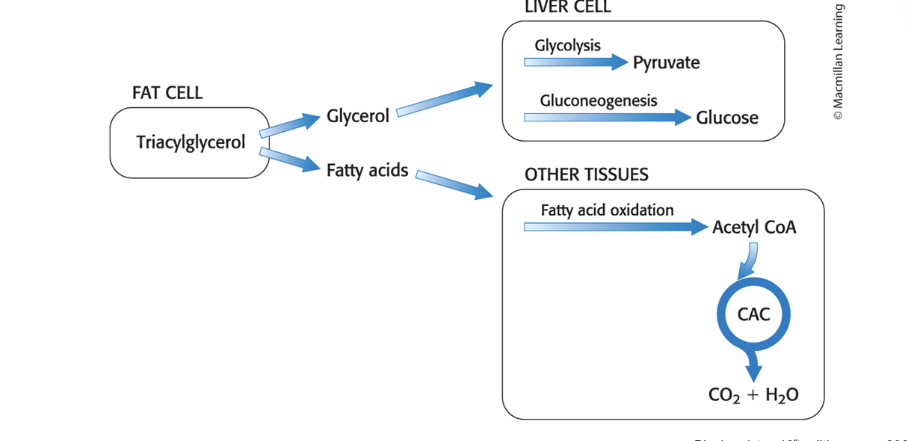
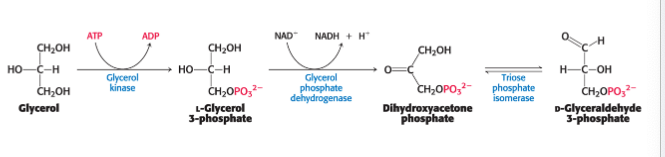
glycerol is converted to…
glycolytic intermediate in the liver
glycerol is absorbed by the liver → phosphorylated → oxidized to dihydroxyacetone phosphate and isomerized to glyceraldehyde 3-phosphate
fatty acids are transported…
via bloodstream for energy (ATP) in tissues
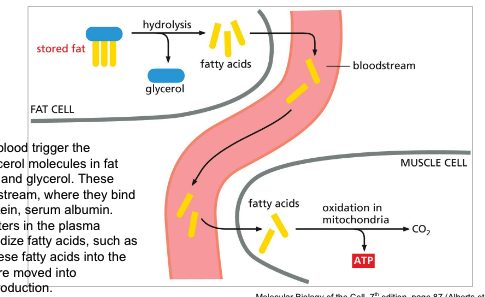
low glucose levels in the blood trigger the hydrolysis of the triacylglycerol molecules in fat droplets to free fatty acids and glycerol
these fatty acids enter the blood stream → bind to abundant blood protein serum albumin
special fatty acid transporters in the plasma membrane of cells oxidize fatty acids, such as muscle cells → pass fatty acids into cytosol → goes into mitochondria for energy production
fat as an energy source
fat is the most efficient energy source
oxidation of a gram of fat releases twice as much energy as oxidation of gram of glycogen
fatty acids provide energy to all tissues in the body, except the brain
brain must rely on circulating glucose or ketone bodies when available b/c fatty acids are poorly utilized by the brain
phospholipids are a…
major class of membrane lipids
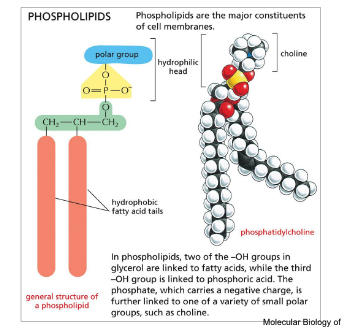
phospholipids = major constituents of cell membranes
hydrophilic head and hydrophobic fatty acid tails
2 of the -OH groups in glycerol are linked to fatty acids while the 3rd -OH group is linked to the phosphoric acid
phosphate carries a negative charge → further linked to one of a variety of small polar groups such as choline
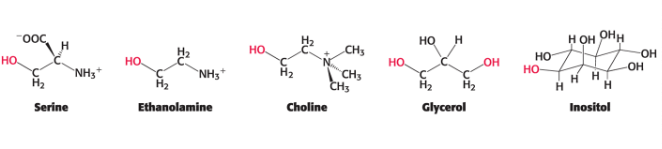
polar groups found in phospholipids
serine
ethanolamine
choline
glycerol
inositol
common phospholipids found in cell membranes
phosphatidylserine
phosphatidylcholine
phosphatidylethanolamine
phosphatidylinositol
diphosphatidylglycerol
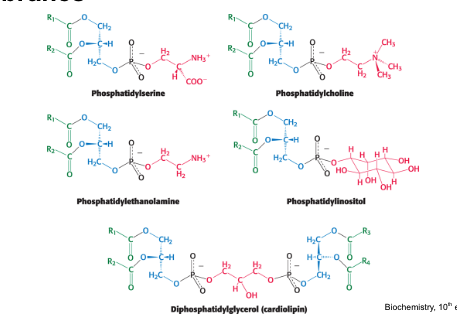
what type of lipid is the following molecule
PC
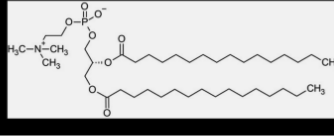
lipid aggregation
based on chemical properties of fatty acids are critical in biology
in water fatty acids can form either a surface film or small, spherical micelles → their derivatives can form larger aggregates held together by hydrophobic forces
triacylglycerols form large spherical fat droplets in the cell cytoplasm
phospholipids and glycolipids form self-sealing lipid bilayers → basis for all cell membranes
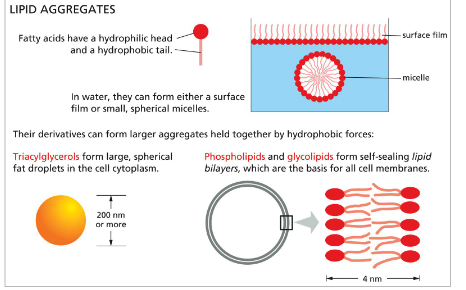
common class of lipids
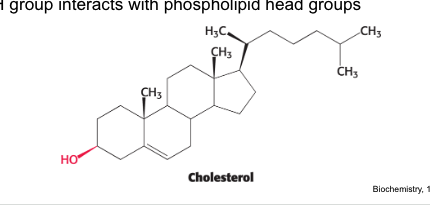
steroids
common multiple-ring structure
cholesterol → found in many cell membranes
testosterone → male sex hormone
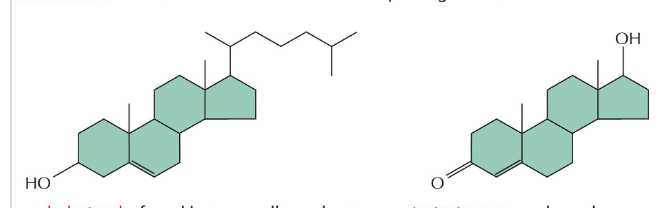
OH group of cholesterol interacts with…
with phospholipids within cell membranes
cholesterol = steroid built from 4 linked hydrocarbon rings
contains a linked hydrocarbon tail at one end and -OH group at the other end
oriented parallel to fatty acid chains of phospholipids in membranes
-OH group interacts with phospholipid head groups