cell cultures
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
cell cultures def
the removal of cells from an animal or plant and their subsequent growth in an artificial environment (also called in vitro
what can we do with the cells once weve taken them out
•Isolate living cells from organisms and keep them alive for days/weeks/months.
•
•We can grow them in suitable conditions and greatly increase their numbers.
•
•Freeze them down and keep them in ‘suspended animation’ for years.
•
•Use the cells as a basis for experimentation – e.g. genetically modify them or test their response to chemicals and drugs.
primary culutred cells def
“freshly” isolated and kept for a short time
e.g. animal or patient’s tissue sample
limited life-span
cell lines cultured cells def
isolated long ago and cloned; immortal
- derived from a single cell of origin (cloned)
able to grow continuously in culture (unlimited life-span)
usually derived from tumours – naturally “immortal” - dividing continuously
Immortality can be also induced (chemical or viral) in primary cells. This process is called “immortalisation”.
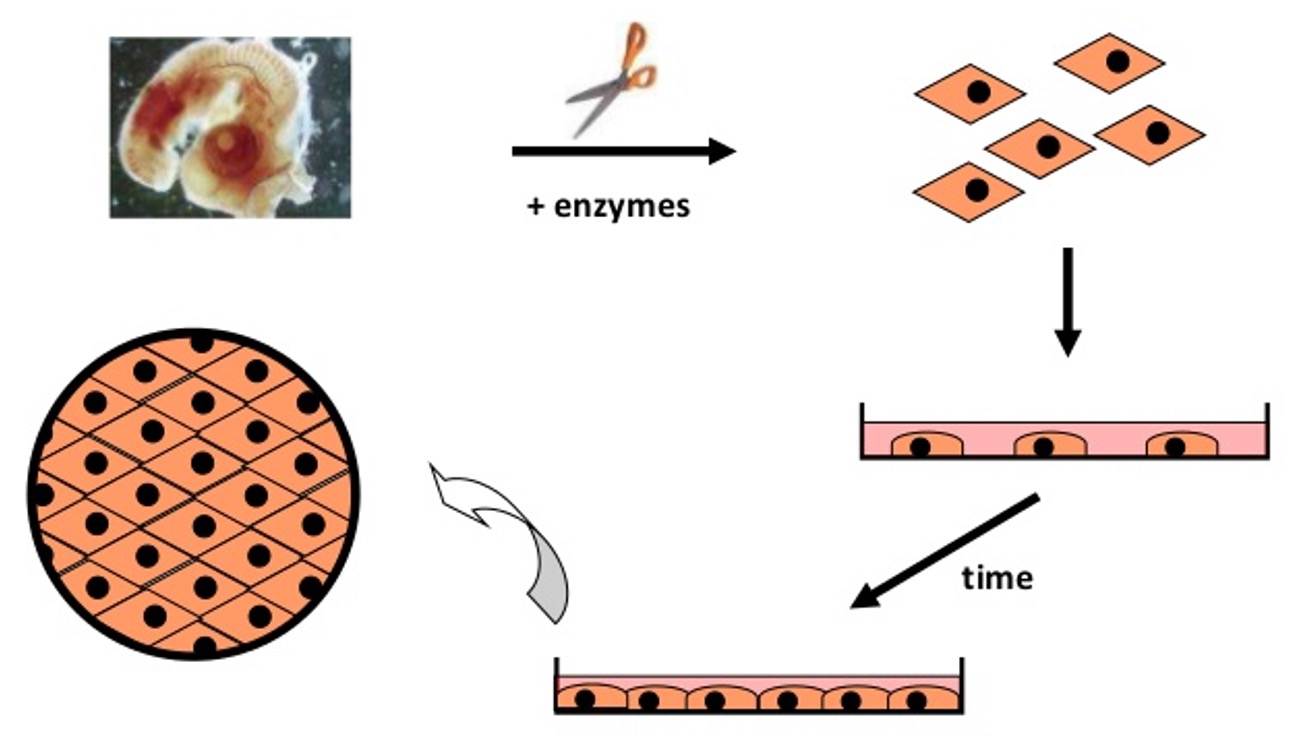
how to obtain cells
•Usually isolated by enzymatic digestion of living tissue, e.g. patient sample.
•
•Perfusion of the tissue with an enzyme, such as collagenase.
•
•Separated cells plated in culture flask.
•
•Special culture medium formulation encouraging proliferation.
commanly used cell lines
HeLa – human, cervical cancer
HEK 293 – human, embryonic kidney
COS-7 – African green monkey, kidney
MDCK-1 – dog, kidney
CHO – Chinese hamster, ovary
1321 N1 – human, glioblastoma
SH-SY5Y – human, neuroblastoma
LNCaP – human, prostate cancer
HL60 – human, acute promyelocytic leukaemia
HUVEC – human, umbilical vein endothelium
HeLa cells – major contribution:
Polio vaccine
First Polio vaccine developed in 1952 by Dr. Jonas Salk
Used HeLa cells from George Otto Gey
HeLa cells: quickly multiplying, robust, susceptible to Polio virus
Used to produce the virus (necessary for vaccine production!) and to test the vaccine
Epidemic stopped following mass vaccination programme
factors that keep cells alive
•a) medium composition and additives
•b) gas phase
c) substrate
Usually each growth container only has a thin film of medium covering the cells – aids gas diffusion
cell culutre medium provides
•
•a stable environment
•adequate nutrients
•correct composition
•correct pH
most media contain
•balanced salt solution
•specific additives
•antibiotic/antifungal agents (optional)
•pH indicator (phenol red)
•We need to add serum purchased separately (usually up to 10%)
media types
•Minimal Essential Medium (MEM)
•Dulbecco’s MEM (DMEM)
•RPMI-1640
•Ham’s medium
basal media
–Keto acids (oxaloacetate, pyruvate)
•Intermediates in Glycolysis/Krebs Cycle
•Maintain maximum cellular metabolism
•
–Carbohydrates (glucose and galactose)
•Energy source
•Low (1g/L) or high (4.5g/L) concentration of sugars
•–Vitamins (riboflavin, thiamine, biotin)
•Precursors for numerous co-factors
•Necessary for cell growth and proliferation
•
–Trace elements
•Zinc, copper, selenium etc.
supplements 5
–L-Glutamine
•Essential amino acid
•Energy source used in protein synthesis
•
•Non-essential amino acids (NEAA)
–Used for protein synthesis
–May reduce metabolic burden on cells
–
–Growth factors and hormones (insulin)
•Stimulate glucose transport and utilisation
•Maintenance of differentiation
•Antibiotics and Antimycotics
–Pen/Strep, gentamicin, amphotericin B
–Reduce the risk of bacterial and fungal contamination
–May lead to antbiotic-resistance or change cells phenotype
–Preferably avoid in long-term culture•Foetal bovine/calf serum (FBS/FCS)
- Growth factors and hormones
- Important for cell attachment
- Variable composition
- Expensive
GAS PHASE
•pH is maintained by the interaction of CO2 (in the gas phase) and HCO3 (in the medium).
•
•We need to pump the CO2 into the incubator.
•
•Most cell culture systems use 5% CO2 in the gas phase to give a pH of 7.3 – 7.4.
•
•Some cell types prefer other %, e.g. embryonic cells – 10% CO2
•
•The cells also need correct temperature = 37°C for mammalian cells.
typical growth conditions
37oC
5% CO2
High humidity
Incubator
Cleaned regularly
growth substrate
used to be glass - in vitro
now plastic
most cell types must adhere to survive and divide
2 main types of cells
adherent cells
non adherent cells
adherent cells
(anchorage-dependent growth)
Most cell types, e.g. muscle, neurons, epithelial, endothelial, etc
monolayer
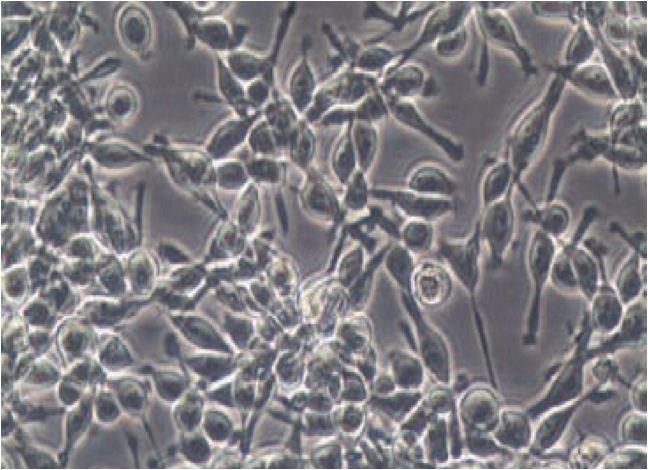
non adherent cells
anchorage-independent growth
immune cells
suspension
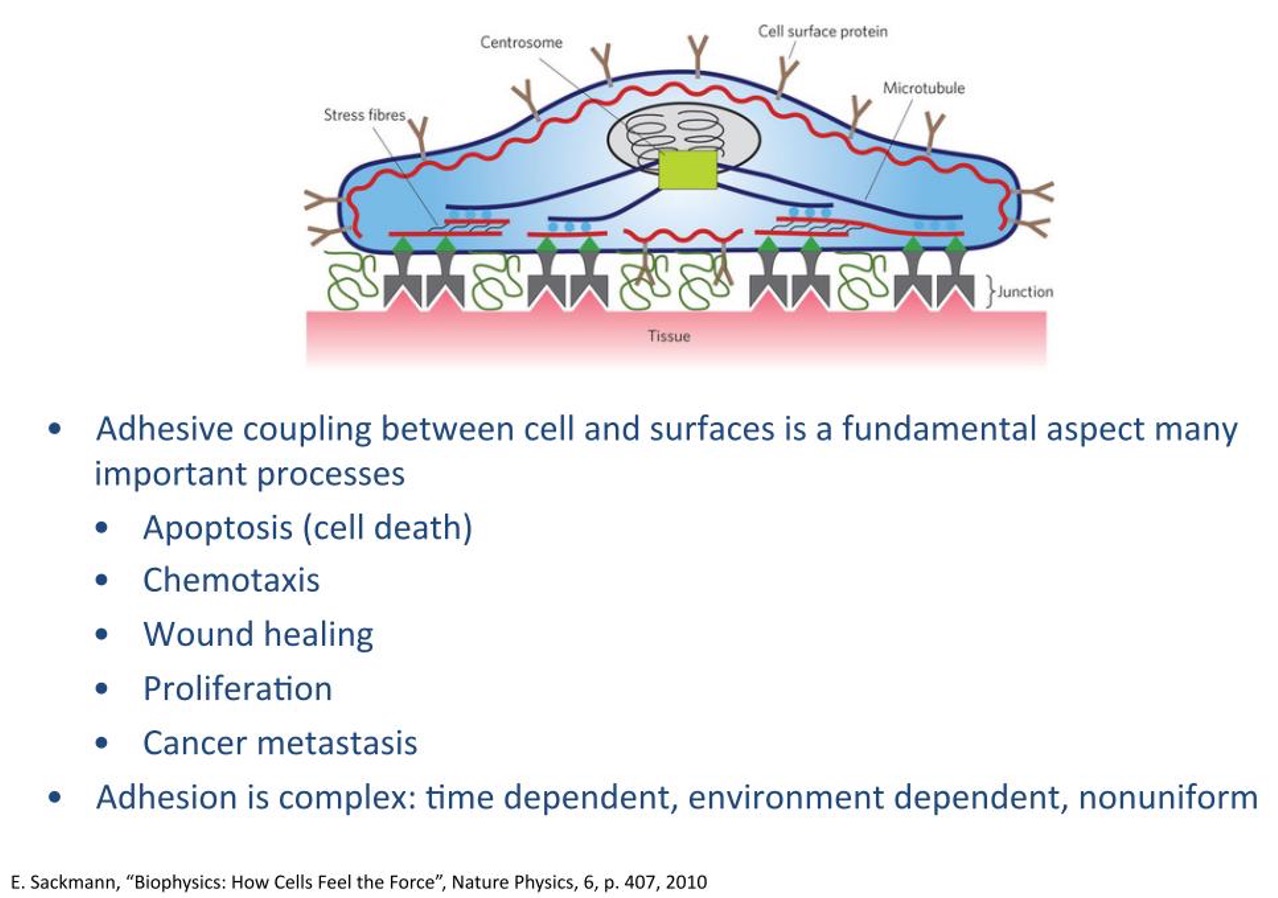
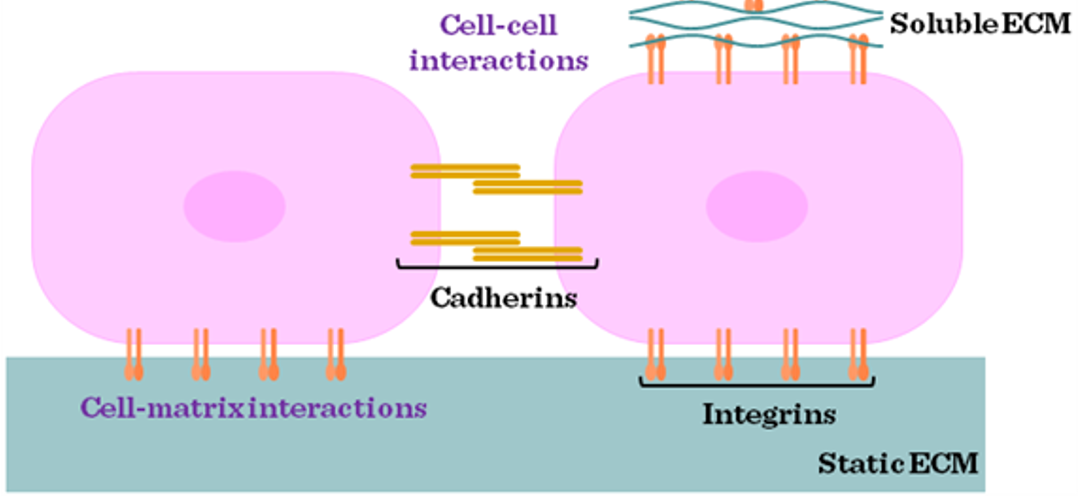
cell adhesion mediated by ..
mediated by cell surface receptors and the extracellular matrix;
–Matrix proteins are secreted by cells, attach to charged plastic;
–Cell surface proteins bind to matrix.
cell adhesion in vivo
contact inibition =
cells stop dividing when too tight (in vitro & in vivo)
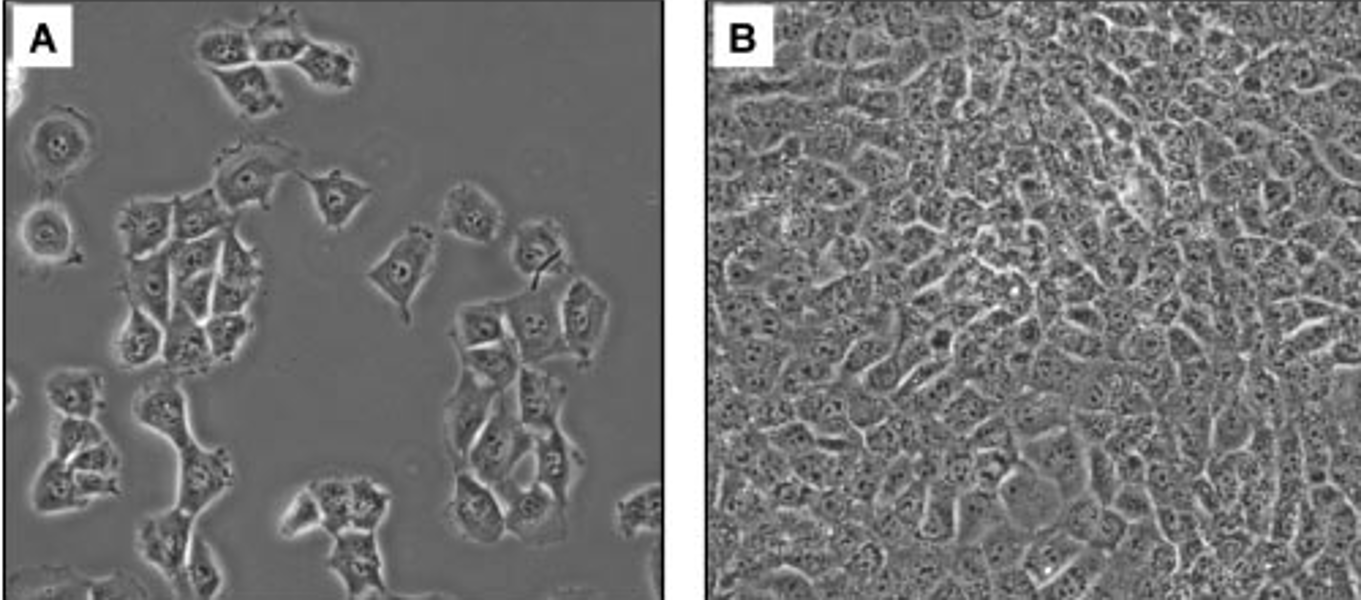
cell density
sparse culture - A
confluent cells - B
growth in vitro
•Lag phase approx. 24 hr
•period of exponential growth
•period of reduced growth
•period of decline*
*Will lead to culture death if
not addressed.
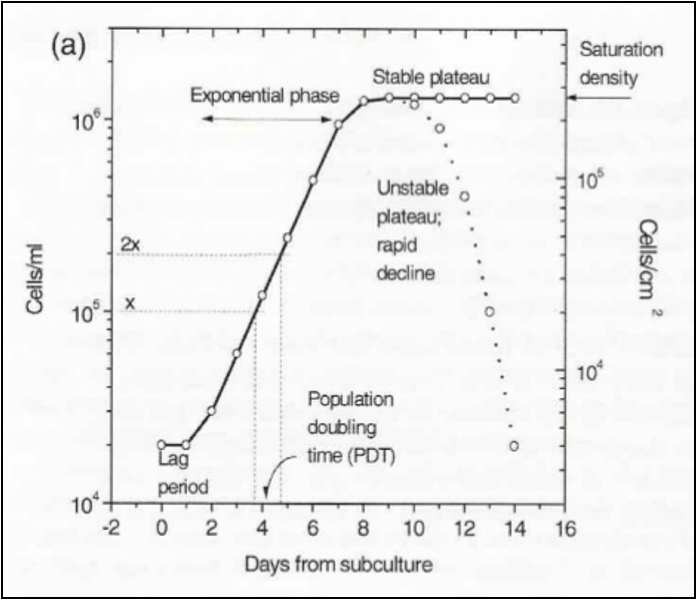
cell passaging def
transferring a portion of cells into a new flask
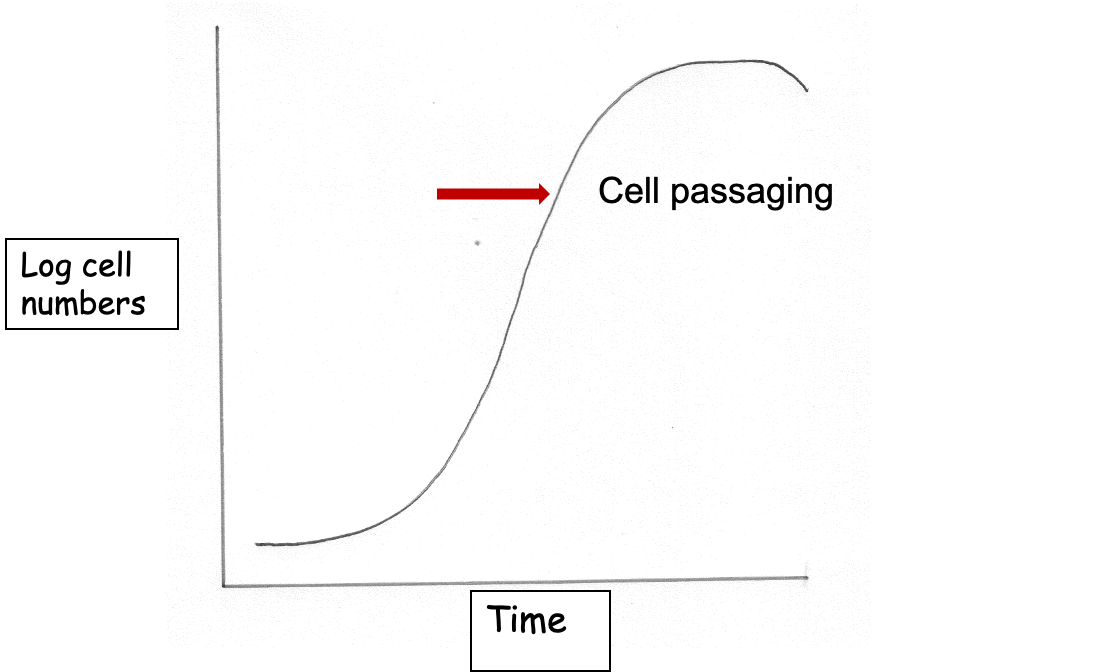
growth curves
Establishing how quickly cells grow is a fundamental observation in cell culture
characteristic of a particular cell line under certain defined conditions,
allows the effects of a variety of agents to be tested,
indicates if problems have occurred in the culture,
allows future cell usage to be estimated.
Growth rate depends on the cell type and initial cell density
why we preserve cells
Storing of human sperm/oocytes or even whole embryos – IVF.
Preservation of endangered species
•Excess cells can be stored for later use, can also be used in the event of a disaster (forms a reference stock).
•
•Cells can be donated to other researchers.
cryopreservation
•Cryo means ‘cold’
•
•Cells in -80°C freezer for short periods
•
•Cells in -196 °C liquid nitrogen – indefinitely
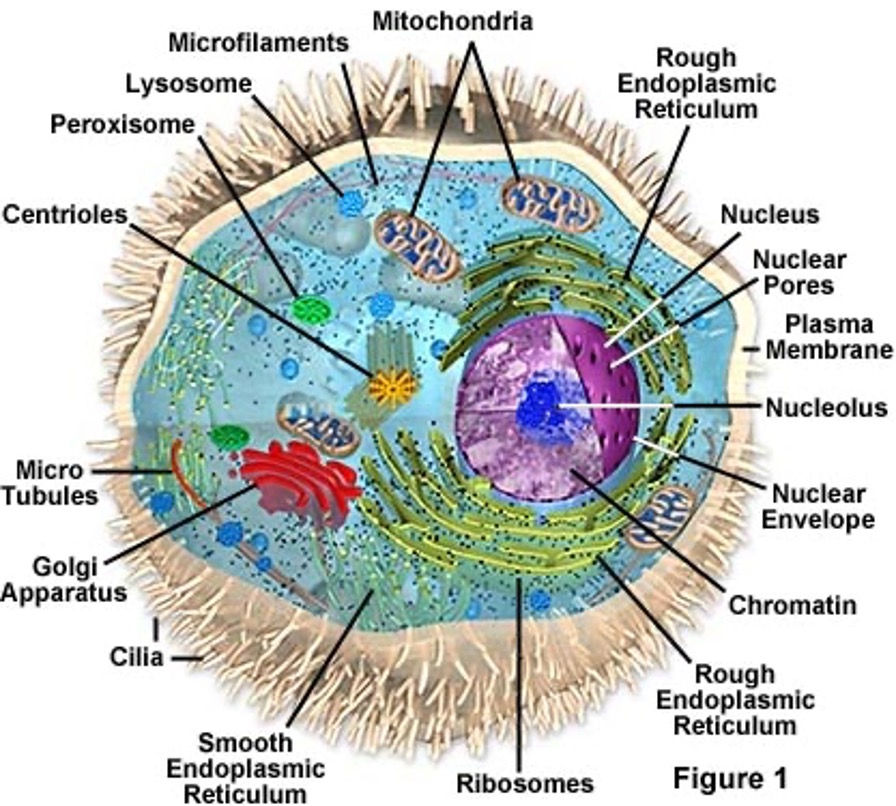
Components of the cell
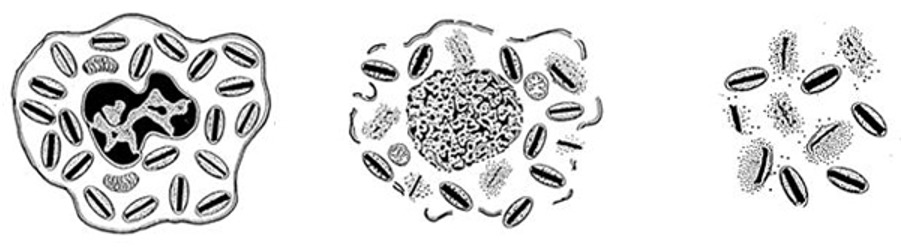
issues with preserving in ice
•Biggest problem is intracellular ice formation:
- causes severe disruption to cell membranes (and organelles).
•
•Not apparent on freezing – but on thawing very poor (or practically zero) cell viability.
•Intracellular ice formation is minimised by using a cryoprotectant
•
•Most commonly used is DMSO (dimethylsulphoxide)
For successful freezing we need
ØFreezing medium = normal culture medium + 10% DMSO*
ØCRYOVIALS
ØHealthy cells in log phase of growth, without contaminations, ca. 100% viability
Can’t exceed 10%, as DMSO is toxic to cells!
freezing protocol
1. Harvest cells (count + asses viability).
2. Resuspend in freezing medium and place in cryovials.
3. Gradual and controlled rate of cooling*
cell thawing process
…opposite to freezing: we want to warm cells up as soon as possible
•Take one frozen vial and place in warm (37°C) water bath,
•When thawed, centrifuge the cells to remove the DMSO*,
Resuspendin normalwarmmedium and place in a flask
after thawing
•The growth rate is often quite slow for a few of days.
•
•Subsequent flasks should show normal growth rates.
•
Usual checks, on growth rate, morphology and viability should be undertaken
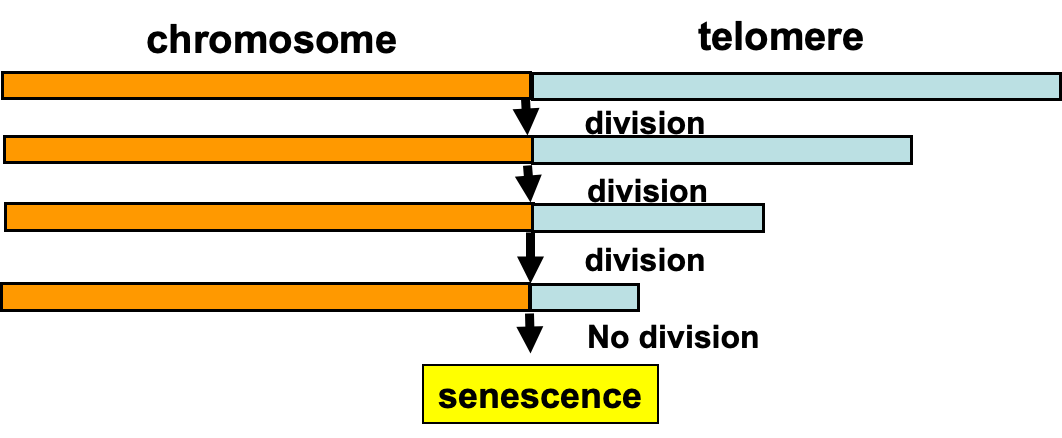
replicative senescence
Human fibroblasts in culture don’t divide more than 50 times
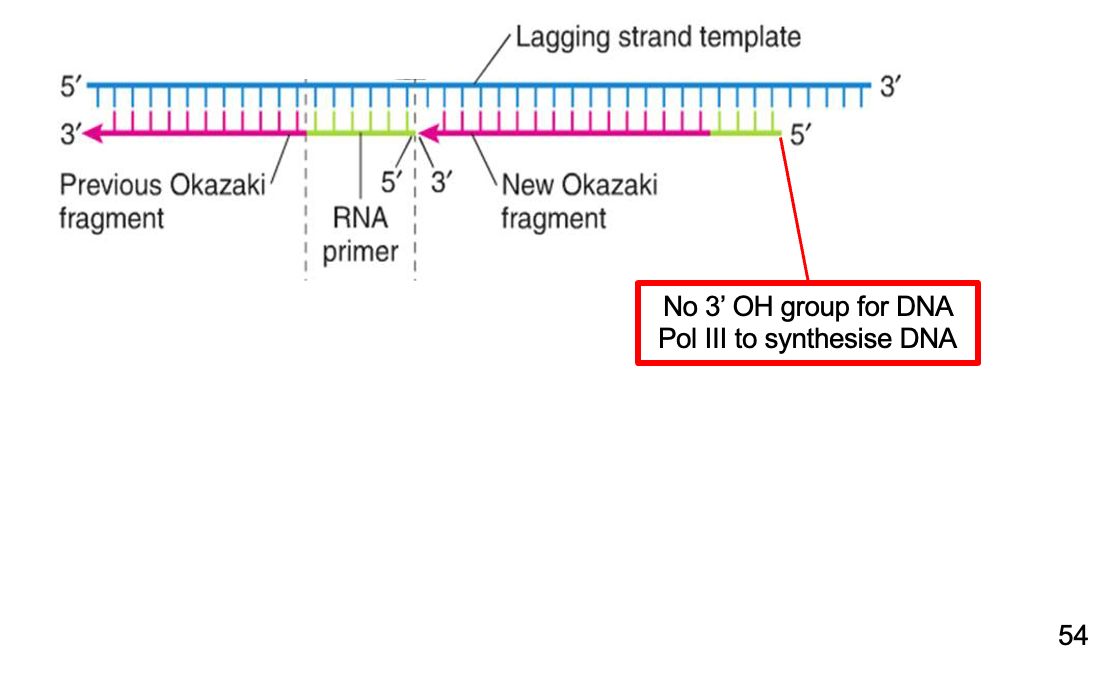
end replication problem
RNA primer removal leaves a gap on lagging stand – shortens chromosome!
telomeres properties
repeated DNA sequences [(TTAGGG)n in humans]
5000-15000 base pairs
protect chromosome ends from exonucleases
facilitate complete replication of chromosomes
promote chromosomes pairing during meiosis
![<p><span>repeated DNA sequences [(TTAGGG)n in humans]</span></p><p style="text-align: left"><span> 5000-15000 base pairs</span></p><p style="text-align: left"><span> protect chromosome ends from exonucleases</span></p><p style="text-align: left"><span> facilitate complete replication of chromosomes</span></p><p style="text-align: left"><span> promote chromosomes pairing during meiosis</span></p>](https://knowt-user-attachments.s3.amazonaws.com/79a4b788-6b57-4c29-917a-b05d9fab6dc8.jpg)
Telomeres – the mitotic clock
In normal somatic cells, telomeres lose 50-250 base pairs upon each cell division.
Telomere shortening is the intrinsic timing mechanism that limits the number of cell divisions
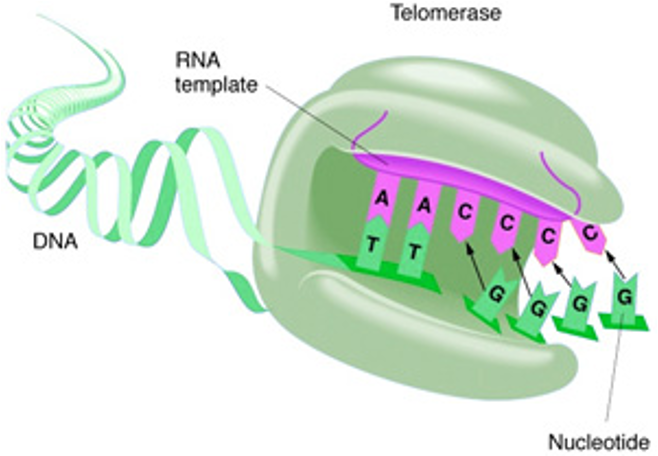
telomerase
RNA à DNA polymerase that adds telomere repeat segments after replication;
Absent in normal somatic cells;
Expressed in stem cells and germline cells – these retain the ability to divide!
Expressed also in cancer
or immortalised cells.