L4 - Protein Synthesis and Structure
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
All proteins function by ______ interactions with other molecules. A protein’s ability to interact with other molecules determines its biological properties.
specific
How do proteins achieve specificity?
Through evolved protein structure like its shape and chemical properties of that shape.
What are the monomers of proteins?
Amino acids
Amino acid structure
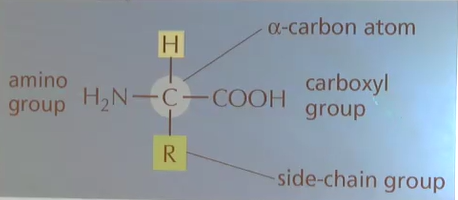
What region of an amino acid varies between amino acids?
Side-chain group (R-group)
At pH 7, both the amino acid and carboxyl groups are ______.
ionized
What charge does the amino group of an amino acid carry in solution/cells?
positive (addition of H)
What charge does the carboxyl group of an amino acid carry in solution/cells?
negative
Properties of side chains
acidic polar (- charge), hydrophilic
basic polar (+ charge), hydrophilic
uncharged polar, hydrophilic
nonpolar, hydrophobic
hydrophilic
water-loving
hydrophobic
water-fearing
Amino acids are linked together by ____ ____.
Peptide bonds
Peptide bonds
Condensation reaction that links together amino acids, release of a water molecule occurs.
Small # vs. Large # of amino acids linked together
Small # = peptide
Large # = polypeptide
Polypeptide chains have polarity, created by the _____ and _____ ends of the chain.
Amino terminus (N-terminus) → + charge; Carboxyl terminus (C-terminus)→ - charge
What is the convention to write sequence in a polypeptide chain?
N-terminus to C-terminus
Codon
A sequence of 3 nucleotides that codes for one amino acid.
Amino acids can have multiple codons that code for them (64 codons vs. 20 amino acids)
the codon to amino acid translation is called the “genetic code.”
How many possible codons are there?
4 × 4 × 4 = 64 possible codons
How many possible “reading frames” for an RNA molecule are there?
3

What is the start codon that defines the reading fame?
AUG
Can also encode methionine within a protein
What are the 3 stop codons?
UAA, UAG, UGA → only encodes for stop
mRNA is translated in the ___ to ___ direction, and polypeptides are synthesized in the ____ to ____ direction.
5’ to 3’ direction; N-terminus (NH2-) to C-terminus (-COOH) direction
ORF
Open Reading Frame, part of the mRNA that actually encodes for the amino acids in the protein.
tRNA
RNA that act as adaptors to translate the mRNA code and bring in appropriate amino acids.
Carries amino acids and complementary anticodons that pair to mRNA in antiparallel fashion.
Ribosome
A machine that will align the mRNA sequence with the adaptors and then link the amino acids together to form the polypeptide chain.
Carries amino acid on mm
To which part of the tRNA is an amino acid attached?
The correct amino acids together is attached to the 3’ end by tRNA synthase (an enzyme)
tRNA structure
“Clover-leaf” structure
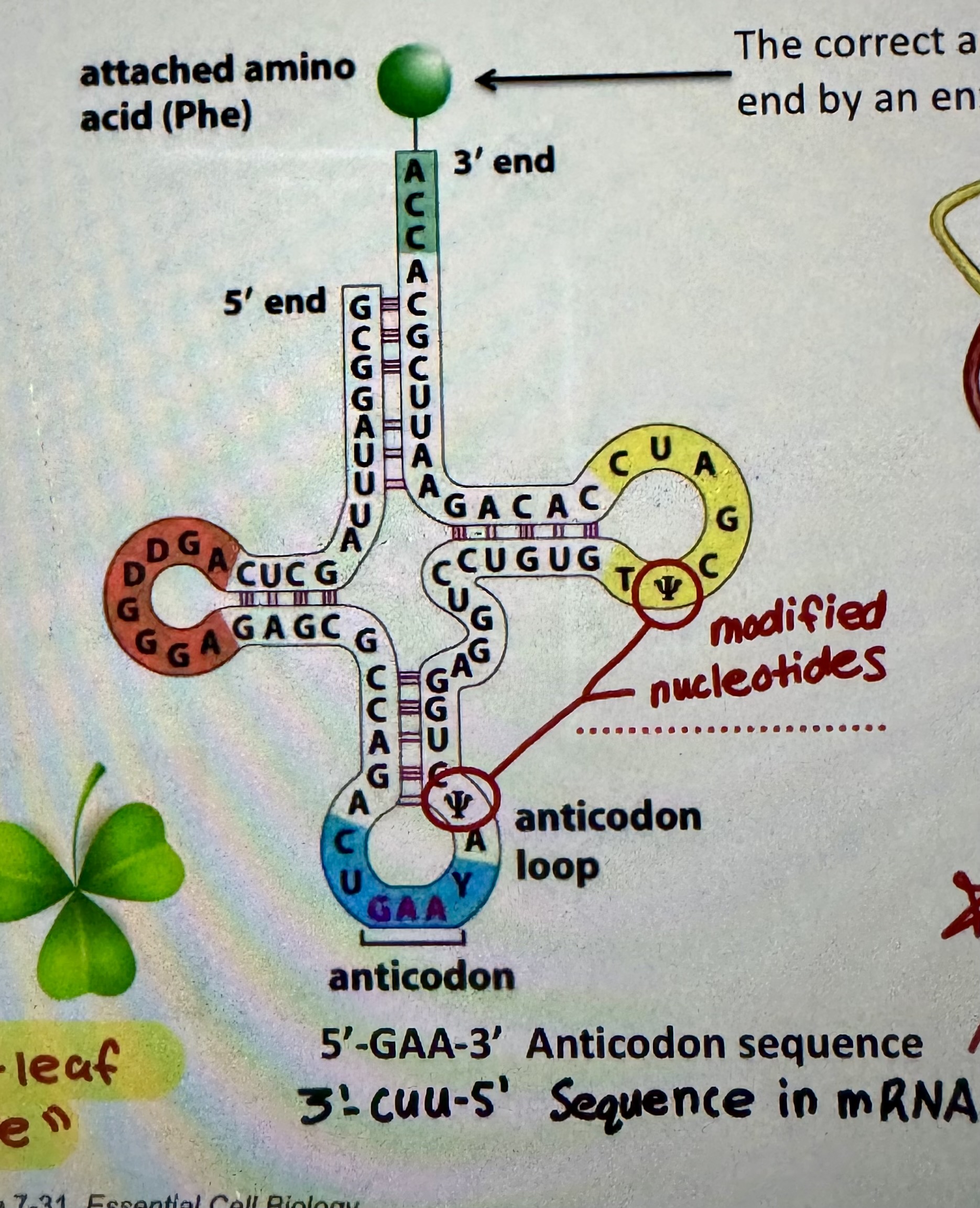
tRNA synthetase
tRNA bind to a specific anticodon sequence in tRNA and bind to specific amino acid. And link them together.
There is at least ___ tRNA synthetase for each amino acid.
1; each tRNA synthetase is specific for 1 amino acid and the appropriate tRNA(s)
small ribosomal subunit
Binds mRNA and matches tRNAs to mRNA codons.
large ribosomal subunit
catalyzes peptide bond formation (RNA acts as catalyst)
E-site on Ribosome
Exit
P-site on Ribosome
Peptidyl-tRNA
A-site on Ribosome
Aminoacyl-tRNA
Steps to Translation
New tRNA binds to A-site
Peptide bond forms between new amino acid and polypeptide chain
Large ribosomal subunit translocates, moving new tRNA (with chain attached) to P-site, and old tRNA to E-site
Small ribosomal subunit translocates to complete this action
old tRNA ejected
Cycle repeats with newly bound tRNA.
Steps to translation inititation
Translation initation factors + initiator tRNA (carrying methionine) bind to small ribosomal subunit
mRNA binds to small ribosomal subunit
Small ribosomal unit, with bound initiator tRNA, moves along mRNA searching for first AUG
once AUG found, translation initiation factors dissociate, and large ribosomal subunit binds. tRNA is in P-site
Steps to Translation end
Stop codon in A-site triggers binding of release factor
Polypeptide chain released (addition of water molecule)
Ribosome dissociates
Primary structure of a protein
the amino acid sequence
Secondary structure of a protein
The path of polymer backbone (ignoring side-chains), includes alpha-helices and beta-sheets.
Tertiary structure of a protein
The overall structure of 1 polypeptide including all side-chains (the positions of all the atoms in the polypeptide, aka the 3D structure)
Quaternary structure of a protein
the overall (3D) structure of a multi-subunit protein (some proteins are made of >1 polypeptide bound tightly together)
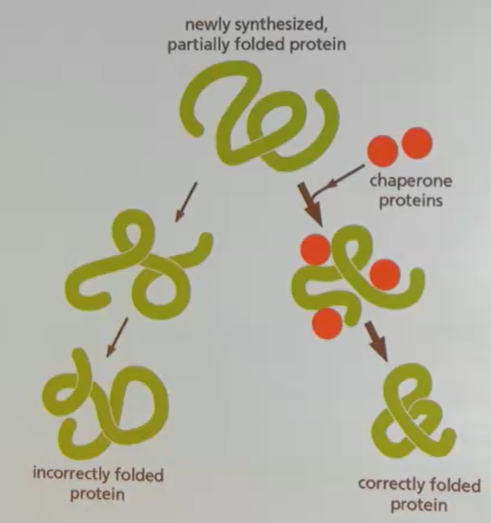
Is a denatured/unfolded protein functional?
No!
What did the Anfinsen Experiment (where protein was denatured from exposure to high con. of urea, and then taken out of urea where is reformed) tell us about proteins?
The protein reformed and gained its function after being removed form urea, thus, all of the information a protein needs to encode for its shape/structure is present in its primary structure (aka. amino acid sequence).
What type of proteins assists a majority of proteins to fold and how?
Chaperone proteins; they often use ATP hydrolysis as an energy source to help fold other proteins.
Are peptide bonds covalent or noncovalent?
Covalent
What are the 4 types of noncovalent interactions that help determine the 3D structure of a protein, and why do they occur?
van der Waals interactions
electrostatic interactions
hydrogen bonds
hydrophobic interactions
These interactions occur because it provides a minimum energy state for the protein (all of the atoms in the protein are happy!), this is favorable. All of these interaction are optimized.
What type of bond occurs between the alpha-carbon and side-chain of an amino acid?
Covalent bond
In general, what makes something hydrophobic?
It is made out of mostly carbon and hydrogen
It is unable to make favorable interactions with water
It displays the hydrophobic effect
In general, what makes something hydrophilic
Inclusion of oxygen and nitrogen in molecule
Favorable intercations with water
Hydrophobic effect
Hydrophobic molecules tend to interact with each other avoid unfavorable interactions with water. This is favorable for water molecules as well because they have less space to avoid on a hydrophobic molecule.
What is the key driver of proteins to fold into compact conformations in aqueous environment?
Hydrophobic interactions. Hydrophobic regions (ie. nonpolar side chains) tend to face inward of compact conformation and interact with each other, whereas as hydrophilic regions (ie. polar side chains) face the outside of the molecule to interact with water.
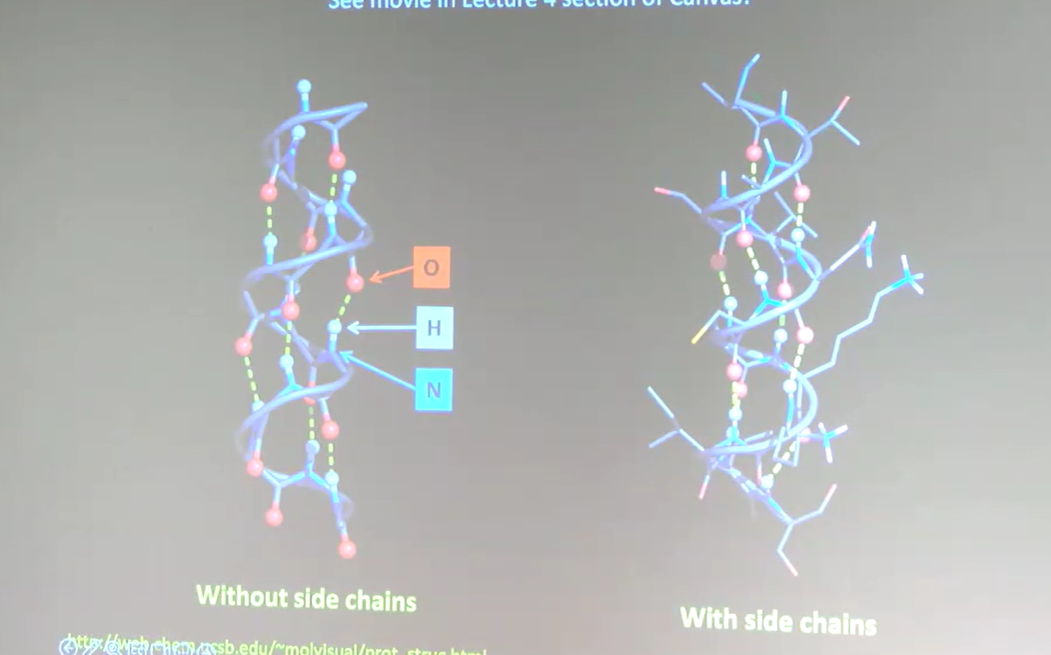
a-helix
Spiral-like (or cylindrical) structure formed from favorable hydrogen bonds created between O, H, and N part of peptide backbone. There are many hydrogen bonds throughout the alpha-helix that allows for the structure. The side chains stick out of the alpha-helix. Part of the secondary structure of a protein.
Beta-sheets
Stretches of polypeptide backbone (strands) form favorable hydrogen-bonds between O, H, N groups side-by-side to form a sheet-like structure. Can be parallel or antiparallel (often signified by arrows). The side chains stick out from the sheets. Part of the secondary structure of a protein.
Protein domain
Segment of a polypeptide chain that can fold independently into a compact, stable structure. In general, each domain has a specific function.
Functionally related protein domains often have very _____ protein sequences.
similar
How may 2 different proteins have similar functionally related domains?
Evolution randomly shuffled domains around to make new genes over millions of years. The ones that do something useful were kept by selection.
How do subunits in the quaternary structure of proteins interact?
Through noncovalent interactions.
Disulfide bonds
Covalent bonds that form between cysteine residues (amino acids) in order to help stabilize favorable protein conformations of secreted proteins. Happens via oxidation (SH group). They only form in oxidizing environments such as the outside of cells, and the inside (lumen) of the ER and other organelles in the secretory pathway and endocytic pathway.
Where CANNOT disulfide bonds form? (Non-oxidative environments)
Cytosol and inside of mitochondria
Example of a protein stabilized by disulfide bonds
Insulin
Example of macromolecular complex that contains proteins and nucleic acids (RNA) + theory it supports
Ribosomes!
Contains 4 RNAs and 82 different proteins. → supports belief that life originated with RNA, being both genetic material and performing all enzymatic reactions.