Part 3: Heating & Cooling Curves
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
What does a heating curve represent?
the change in state and temperature of a substance as it is heated
Draw a heating curve for pure ice
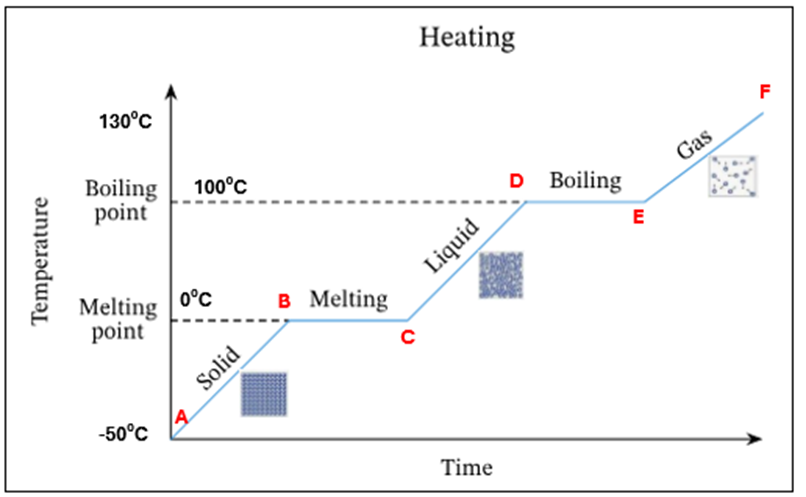
In this heating curve explain what is happening at A
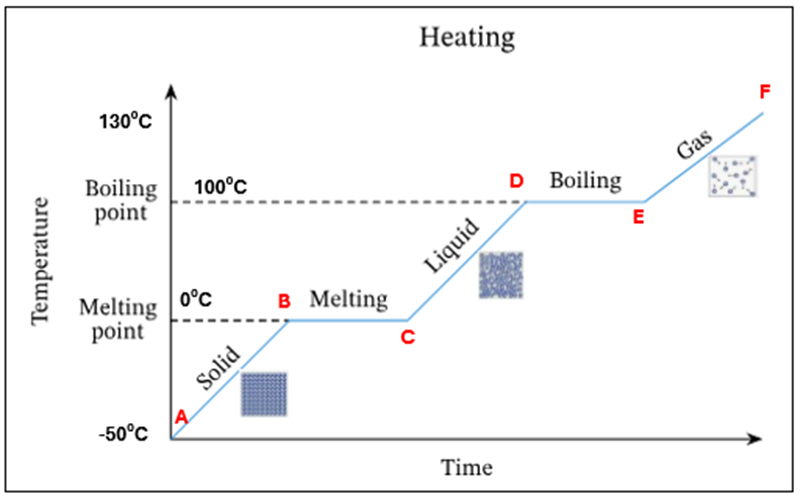
Ice only is present
In this heating curve explain what is happening from A-B
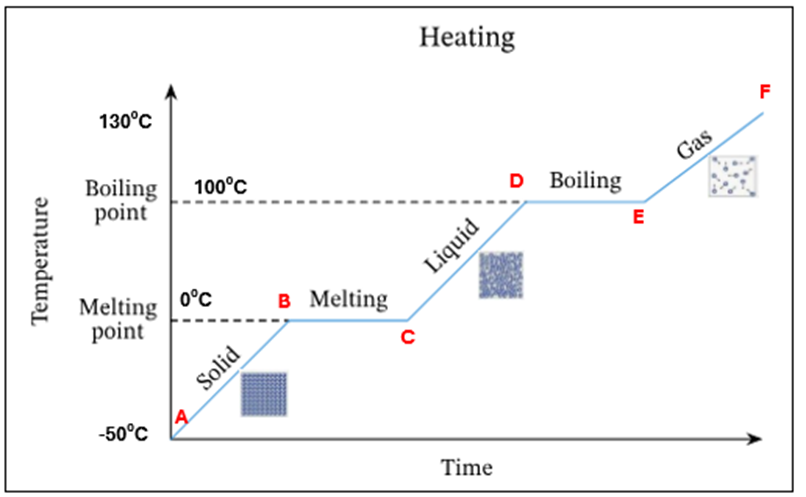
The temperature and K.E of ice is increasing - still solid
In this heating curve what is happening at point B
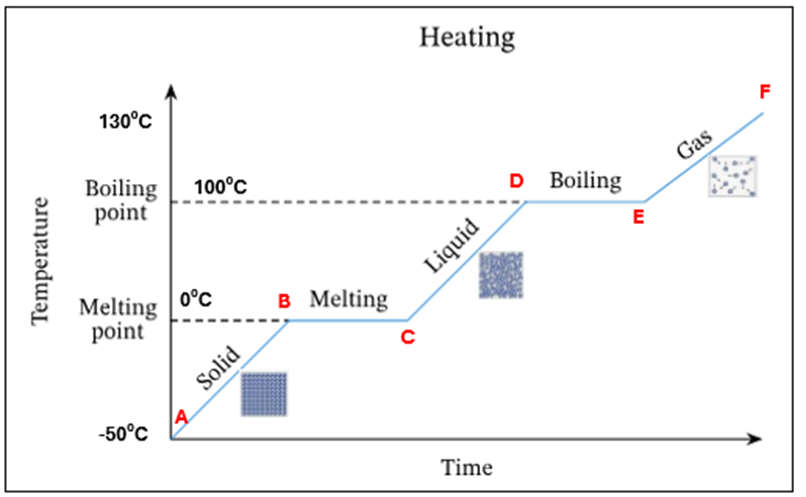
Ice starts to melt at OC - this is its melting point
In this heating curve what is happening at B-C
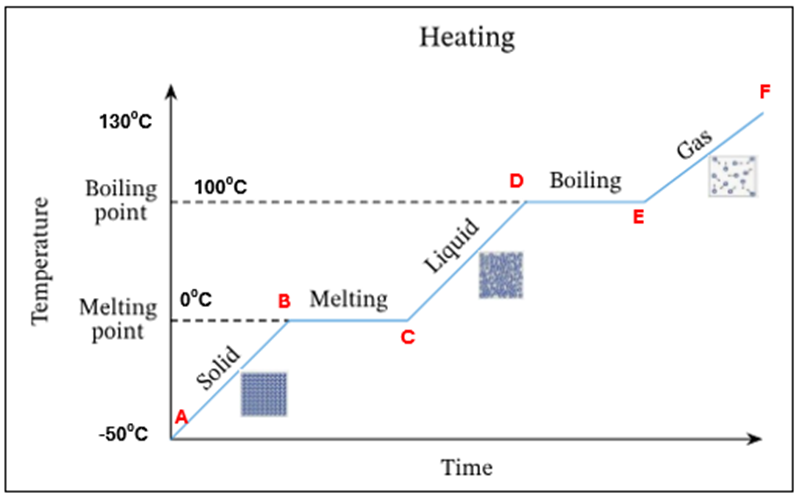
There is a mixture of ice and water present at OC. There is no temperature increase as the energy is being used up to change the state of the substance from a solid to a liquid (*this is known as latent heat)
In this heating curve what is happening at point C
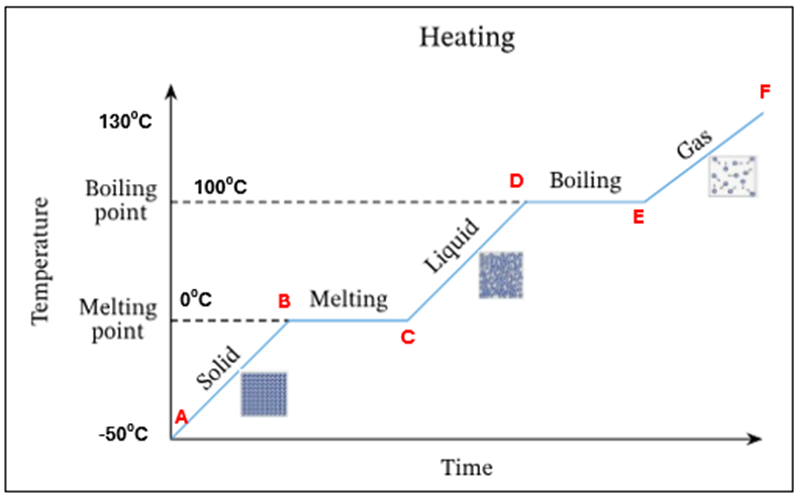
All ice has melted. Only water is present at OC
In this heating curve what is happening at point C-D
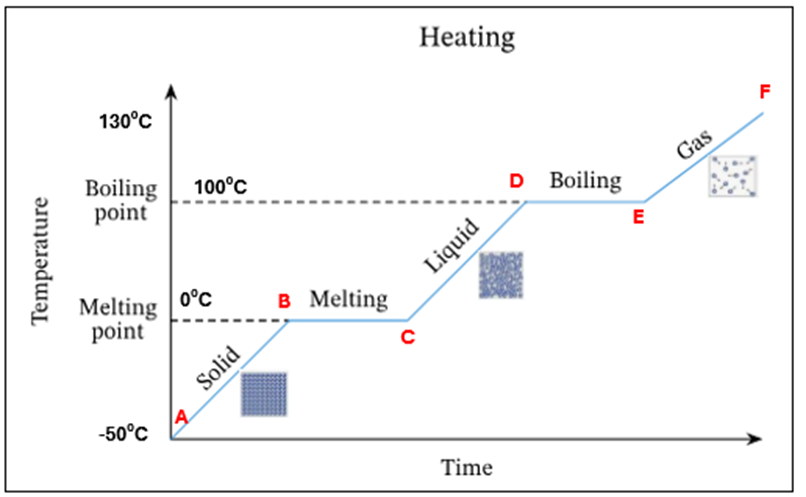
The temperature and K.E of the water is increasing
In this heating curve what is happening at point D
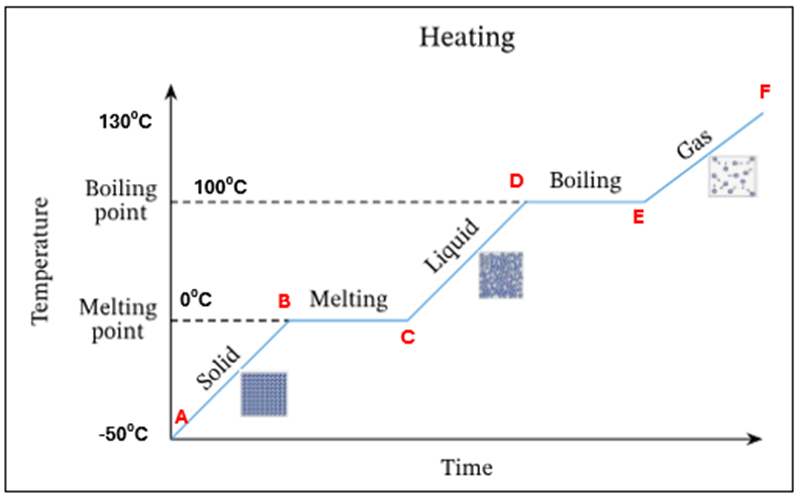
Water starts to evaporate at 100C. This is the boiling point
In this heating curve, what is happening at point D-E
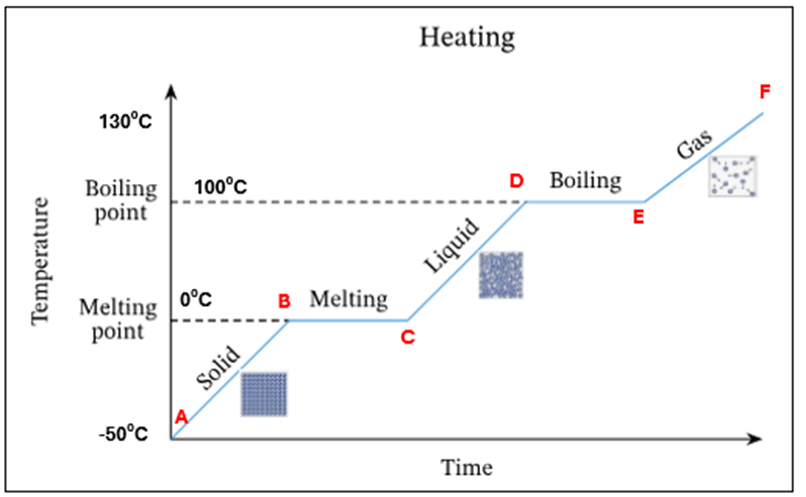
A mixture of water and water vapour (liquid + gas) is present at 100C.There is no temperature increase as the energy is being used up to change the state of the substance from a liquid to a gas (*this is known as latent heat)
In this heating curve explain what is happening at point E
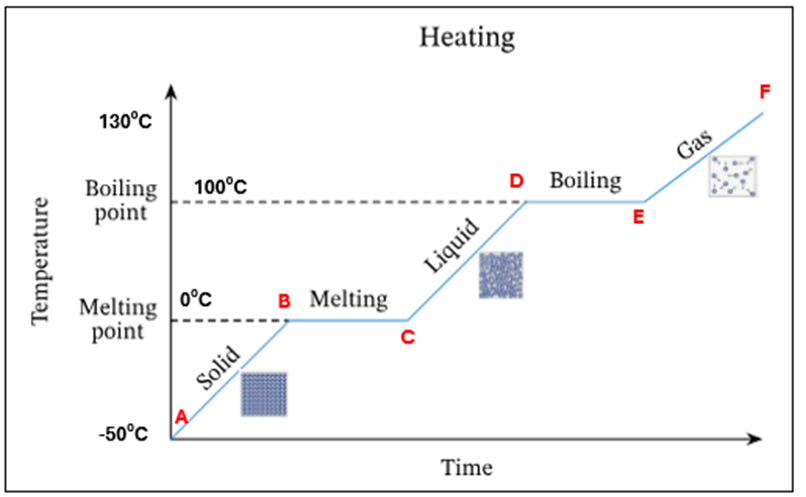
All the water has evaporated. Only water vapour/steam is present at 100C
In this heating curve, explain what is happening at point F
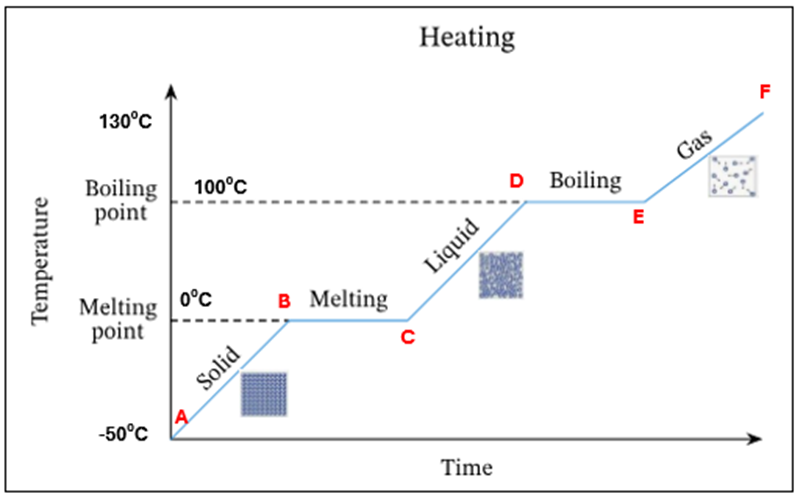
The temperature and K.E of the steam keep increasing
Summary - What is happening at all letters in this heating curve
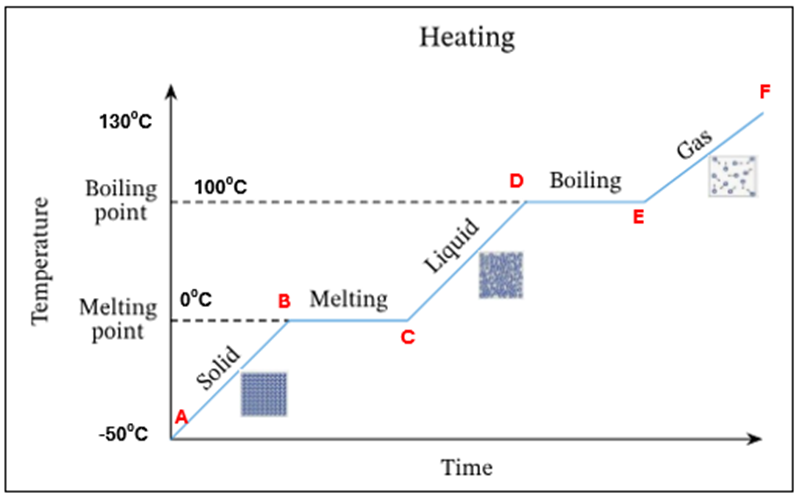
A | Ice only is present |
A-B | The temperature of ice is increasing (increase in K.E) – still solid |
B | Ice starts to melt at O°C – this is its melting point |
B-C | Mixture of ice and water at O°C. There is no temperature increase as the energy is being used up to change the state of the substance from a solid to a liquid (*this is known as latent heat) |
C | All ice has melted. Only water is present at O °C |
C-D | The temperature of water is increasing (increase in K.E) |
D | Water starts to evaporate at 100 °C. This is the boiling point |
D-E | Mixture of water and water vapour present at 100C. There is no temperature increase as the energy is being used up to change the state of the substance from a liquid to a gas (*this is known as latent heat) |
E | All the water has evaporated. Only water vapour present at 100°C |
E-F | The temperature and K.E. of steam keep increasing |
Define LATENT HEAT
the energy absorbed or released by a substance during a change of its state without changing its temperature. Latent means ‘hidden’
What happens to latent heat during heating and cooling?
During heating, latent heat is absorbed to break intermolecular bonds. During cooling, latent heat is released as intermolecular bonds form.
Explain what happens in the different regions of a heating curve (same thing also applies for cooling curves)
Mention what is happening at the
diagonal lines
horizontal lines/plateaus
Diagonal Lines | Show a change in temperature (and K.E.). During these regions only a single state of matter exists, and the sample is getting hotter (colder in the case of cooling curves) |
The plateaus /horizontal lines | These lines mark the phase changes. During these regions, a mix of states of matter exist. The temperature remains constant during these phase transitions because of latent heat. |
What happens to particle energy when temperature increases?
The kinetic energy of the particles increases.
What happens to kinetic energy when the temperature is constant?
Kinetic energy is also constant.
What happens to energy when the temperature is constant but heating continues?
Energy is still being absorbed and is now referred to as latent heat, and that latent heat increases the potential energy of the particles which is used to break the intermolecular forces between particles (not to increase K.E)
Therefore, is breaking bonds an endothermic or exothermic process
Endothermic - because heat (latent heat) needs to be absorbed for the reaction to take place (break/overcome the intermolecular forces)
How does a pure substance change state on a heating or cooling curve?
A pure substance changes state at a single, fixed temperature, so the plateau is a straight, horizontal line (e.g. pure ice melts at 0 °C).
How does an impure substance change state on a heating or cooling curve
An impure substance changes state over a range of temperatures, so the plateau is sloped instead of flat.
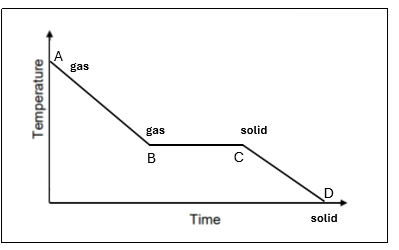
Draw a sketch of a cooling curve for pure ice
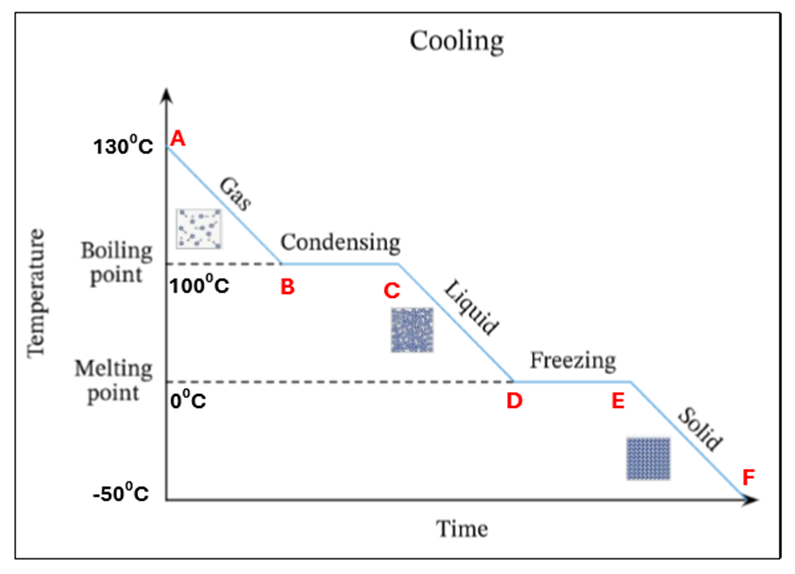
In this cooling curve explain what is happening from points A-F
A | A hot gas only is present |
A-B | The temperature of the gas is decreasing (decrease in K.E) – still solid |
B | The gas starts to condense at 10O°C – this is its condensing point |
B-C | Mixture of gas and liquid at 10O°C. There is no temperature change as the energy being released is used up to change the state of the substance from a gas to a liquid (known latent heat) |
C | All gas has condensed. Only liquid is present at 10O °C |
C-D | The temperature of the liquid is decreasing (decrease in K.E) |
D | Liquid starts to freeze at 100 °C. This is the freezing point |
D-E | Mixture of liquid and solid present at 0°C. There is no temperature change as the energy being released is being used to change the state of the substance from liquid to solid (known as latent heat) |
E | All the liquid has frozen. Only solid ice present at 0°C |
E-F | The temperature and K.E. of the solid (ice) keep decreasing. |
What happens to particle energy when temperature decreases?
The kinetic energy of the particles decreases.
What happens to kinetic energy when the temperature is constant during cooling?
The kinetic energy stays the same.
What happens to energy when the temperature is constant but cooling continues?
Energy is still being released and is now referred to as latent heat, and that latent energy decreases the potential energy of the particles – this then reforms the intermolecular forces (not affecting K.E).
Is forming bonds during cooling an endothermic or exothermic process? Explain.
Forming bonds is an exothermic process because for the intermolecular bonds to be reformed, energy (latent heat) must be released to decrease the P.E. of the particles.
Explain what is different when heating and cooling curves are shown on the same axes
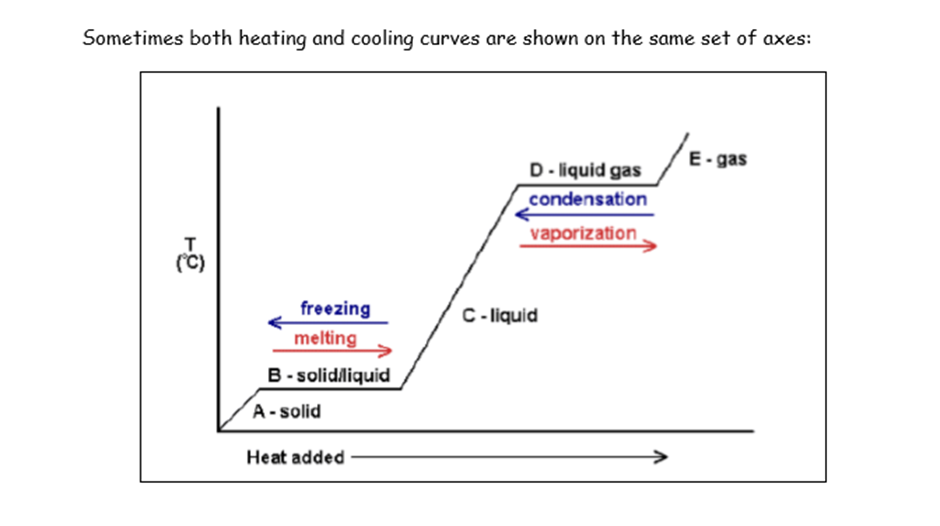
Time is not displayed anymore on the x-axis (instead heat is displayed)
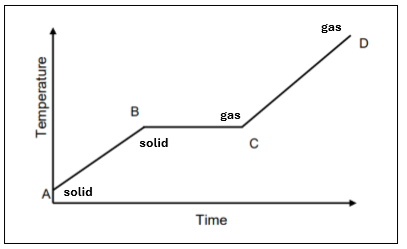
Draw a sketch of a heating curve for a substance that undergoes sublimation
Draw a cooling curve for a substance that undergoes deposition