The Arrhenius Equation
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
10 Terms
What is the Arrhenius equation formula?
k= A x e-Ea/RT
What is ‘k’?
The rate constant- the units depend on the overall order of the reaction.
What is ‘T’?
Temperature, in Kelvins.
K= °C + 273.
What is ‘R’?
The gas constant ( 8.31 J K-1 mol-1 )
What is Ea?
Activation energy- measured in J mol-1
What is ‘A’?
Pre-exponential factor (frequency factor)- no units.
What does the Arrhenius equation show?
It shows how the rate constant k and temperature are related exponentially.
What happens to the value of the rate constant if the activation energy decreases?
The rate constant increases therefore the rate of the reaction increases.
This is because when the activation energy is lowered, more particles have sufficient energy to collide successfully and react.
What happens to the value of the rate constant if the temperature increases?
The rate constant increases therefore the rate of the reaction increases.
When the temperature increases, the particles have more kinetic energy, increasing the likelihood of successful collide with energy equal or greater than the activation energy. As a result, the rate increases.
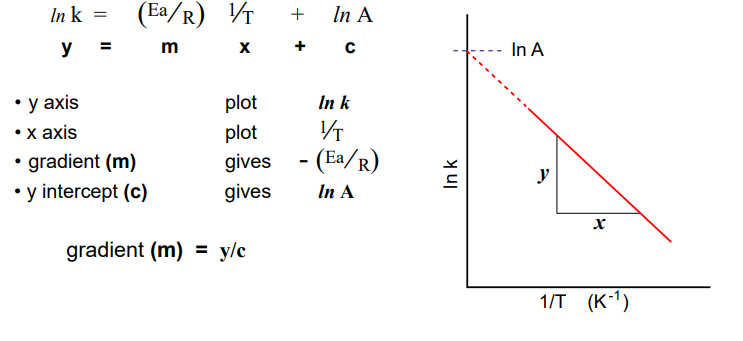
Diagram showing the Arrhenius equation in graphical form.