AP Bio- 1.2
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
what is Water, what is its purpose?
H2O - An important component in many chemical reactions that help organisms maintain homeostasis.
explain water's role in transport
Allows for the transport of materials in different organisms, such as blood in animals and sap in plants.
what is a polar molecule
A molecule with partial charges on the poles that attract to opposite charges on other molecules nearby.
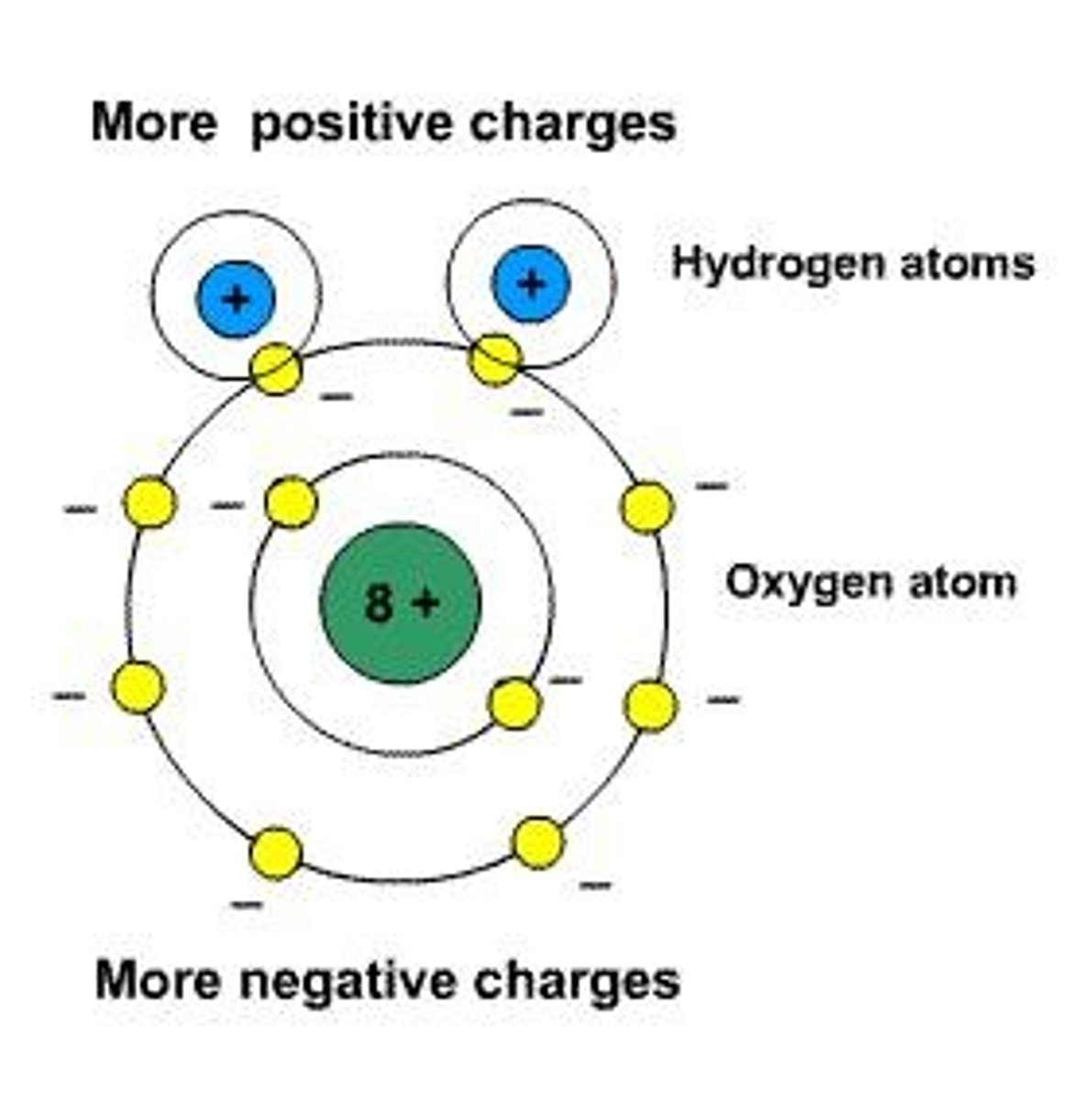
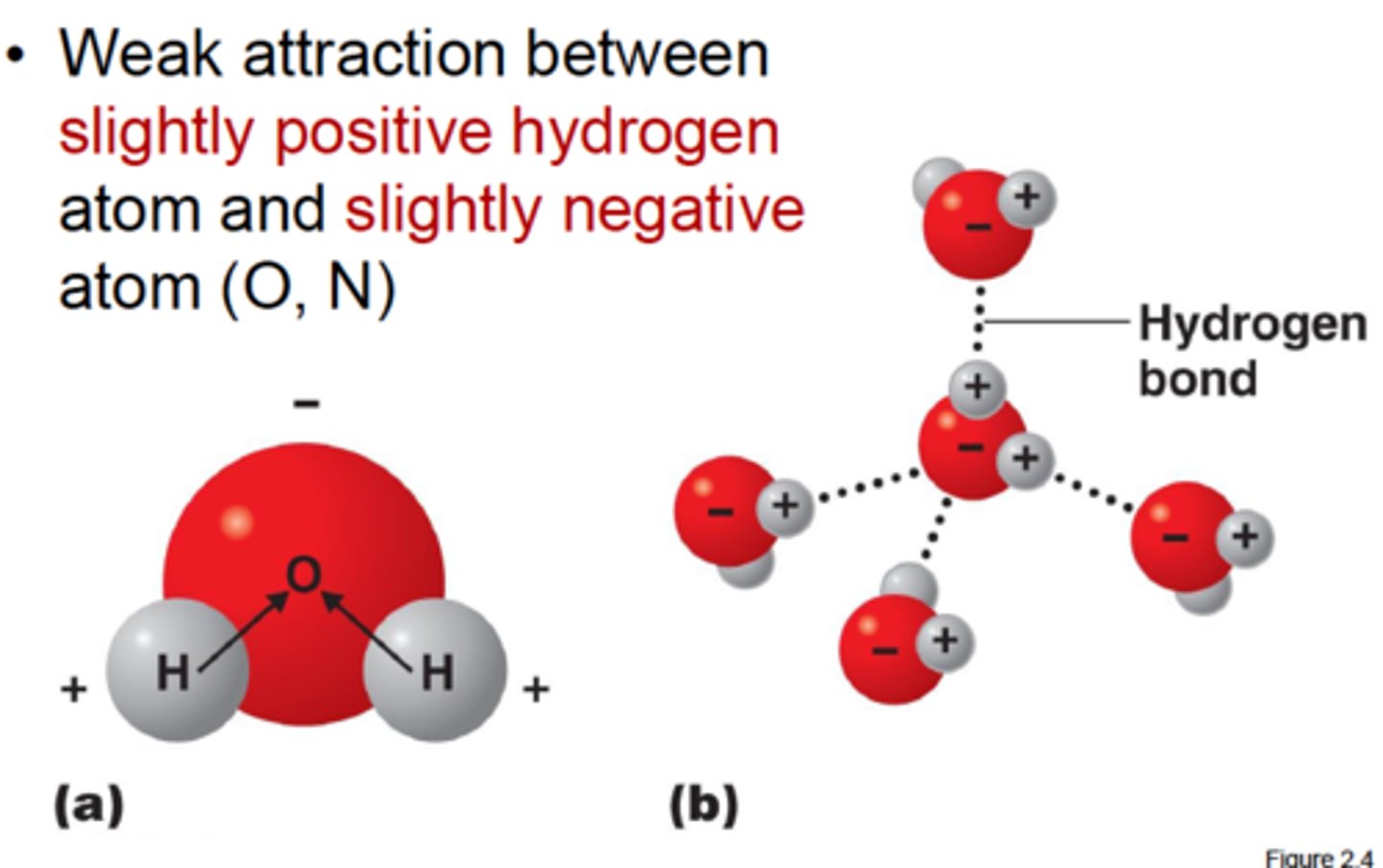
explain hydrogen bonding
a type of weak interaction occurring when a hydrogen atom, that is covalently bonded to a highly electronegative atom, is attracted to another electronegative atom nearby.
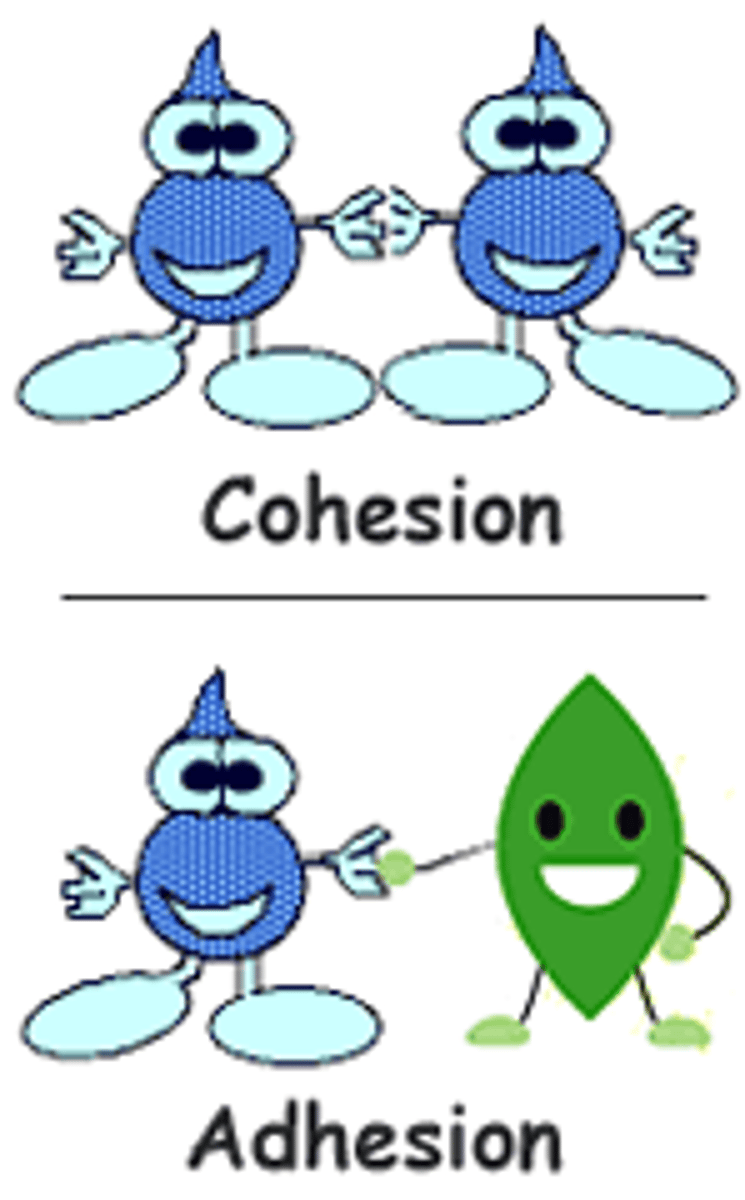
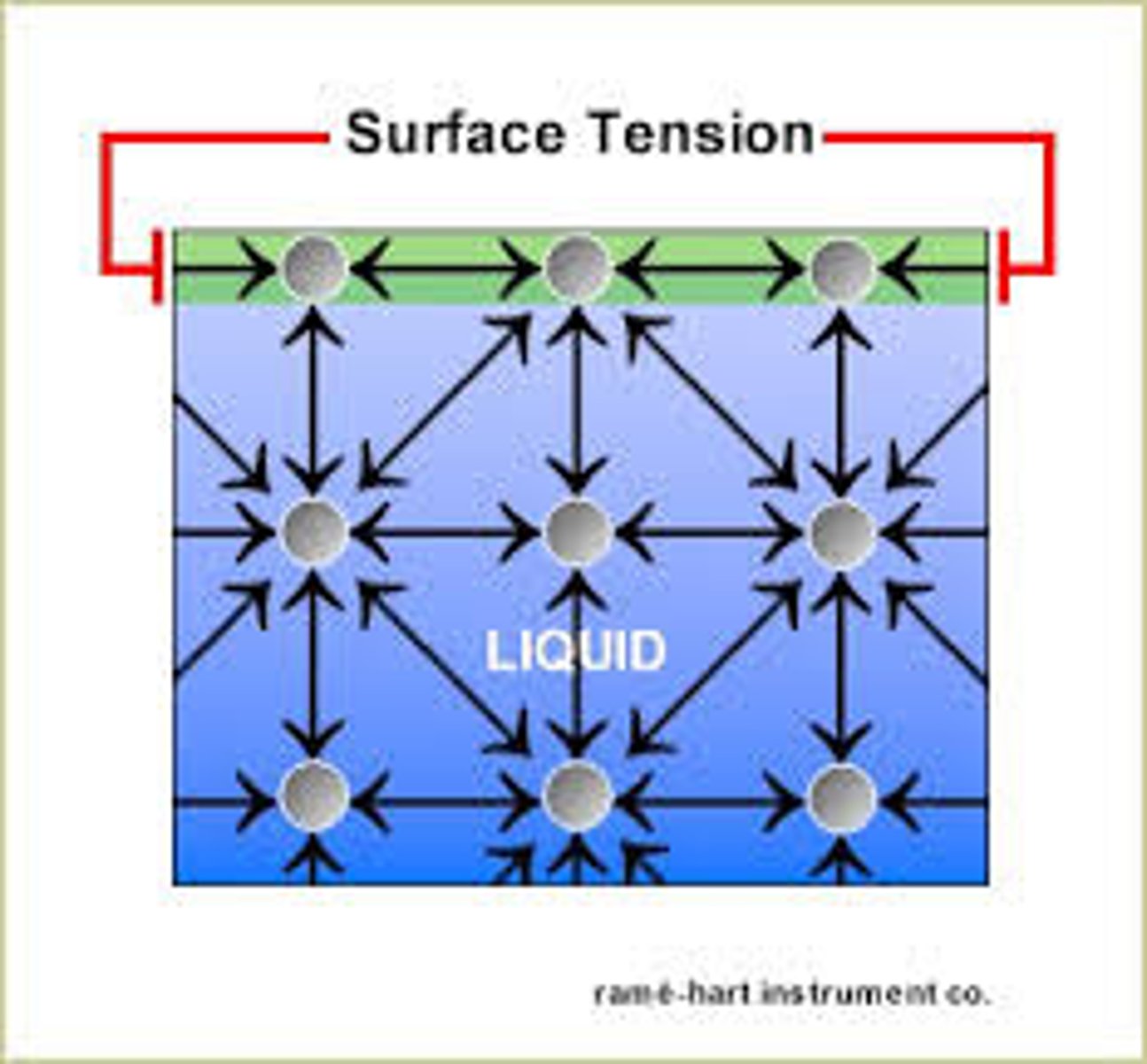
explain cohesion in water
The property that allows water molecules to adhere (stick) to each other,
- results in surface tension, droplets forming
what is the cohesion in water due to?
due to the hydrogen bonding between water molecule
explain adhesion of water
The property that allows water to stick to other molecules and surfaces
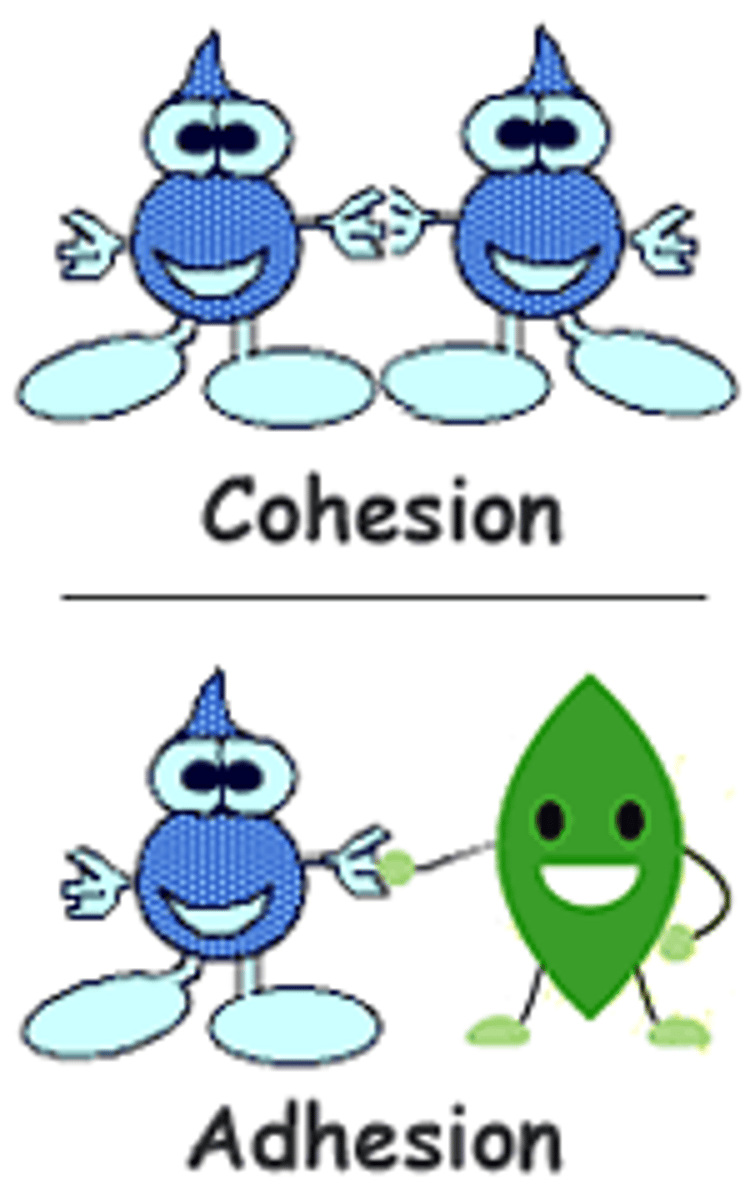
what is adhesion of water due to?
due to water molecule's attraction to other electronegative surfaces
what is surface tension
The cohesive force among water molecules at the surface that resists being ruptured by light objects.
explain Capillary action
The ability of water to adhere to the sides of tubes lined with polar/charged molecules and 'crawl' up the tube due to cohesion and adhesion.
why is capillary action important?
- draws water out of the roots of a plant and up into its stems and leaves
- important in biological systems: helps the movement of fluids in blood vessels, tissues etc.
list the Chemical properties of water
1. Exhibits cohesion and adhesion,
2. has surface tension
3. resist temperature changes
4. acts as an excellent solvent.
Cohesion vs. Adhesion
Cohesion refers to water's attraction to itself, while adhesion refers to water's attraction to other substances.
Water skater bugs
An example of organisms that can float on the surface of water due to surface tension.
explain electronegativity in Water
The Oxygen atom in H2O is more electronegative, causing shared electrons to spend more time closer to the Oxygen, resulting in a negative charge on the Oxygen atom and a positive charge on the Hydrogen atoms.
are hydrogen bonds weak or strong?
Hydrogen bonds are individually weak but collectively strong.
what is needed to break water's hydrogen Bonds, why?
- liquid water has lots of H bonds
-It takes a lot of energy to break all of the hydrogen bonds and cause water to evaporate into steam.
explain what causes water's resistance to temperature Change
Water resists temperature changes due to the energy required to break hydrogen bonds.
why is waters high specific heat capacity useful for organisms?
This property is useful for living organisms that need to maintain a constant internal temperature for homeostasis.
what is a solvent
A solvent is a substance that dissolves other chemicals.
why is water an excellent solvent?
- Water is an excellent solvent because it is polar.
- Very good at pulling ionic compounds (like salt) apart into ions. (which are electrolytes required for life)
explain the water Shell H2O creates around polar molecules
Water can create a water shell around polar molecules that cannot ionize because they are held together by covalent bonds.
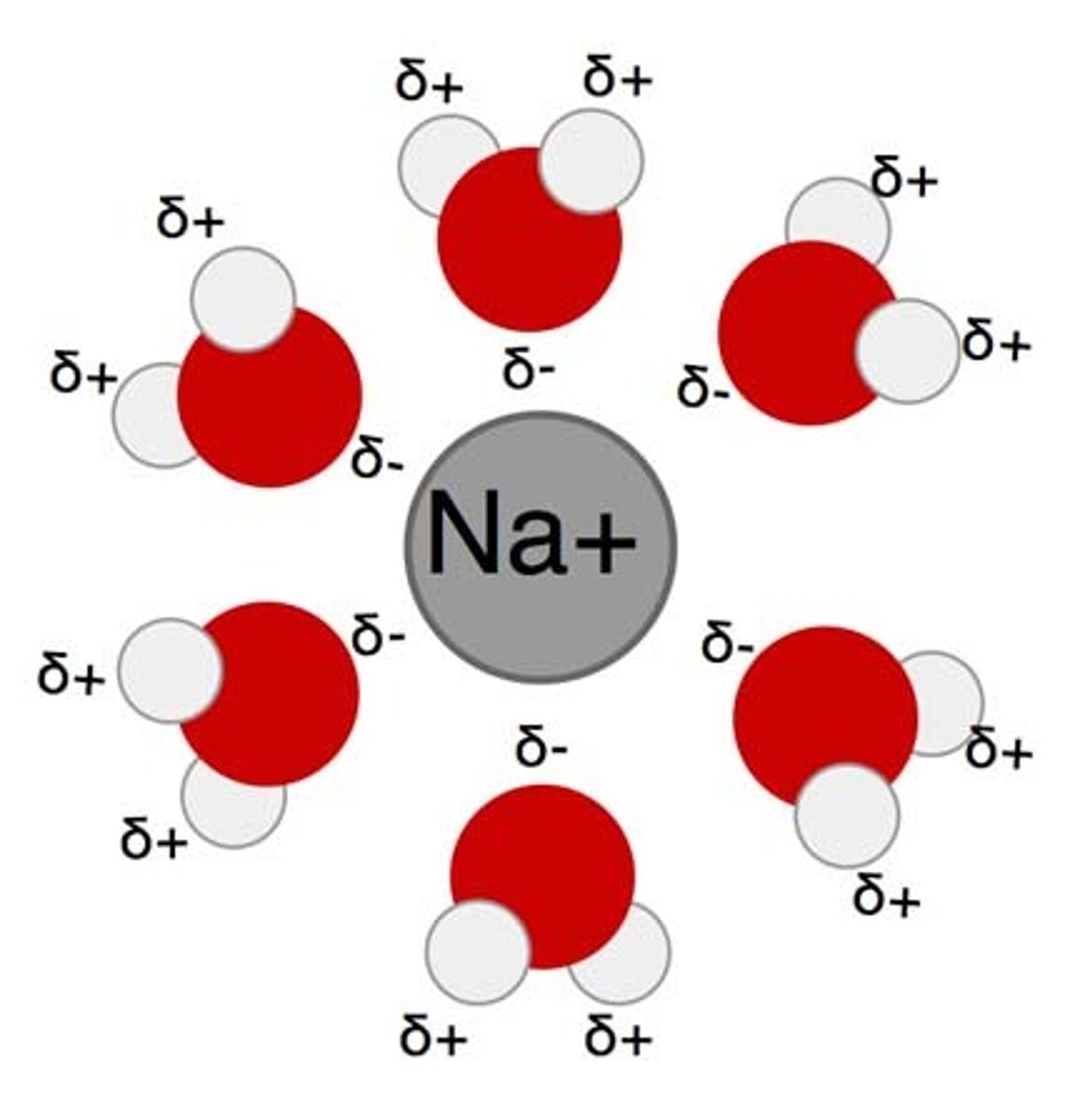
Sugar in Water
Sugar, which we use for cellular respiration, is an example of a polar molecule that can be surrounded by water.