lecture 13 - secondary, tertiary, and quaternary structure
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
what can beta turns do?
allow protein chain to reverse direction
EXAMQ - where is the carbonyl carbon bonded to in the beta turn?
carbonyl C of one residue is H-bonded to the amide proton of a residue three residues away (1st and 4th)
two types differ in orientation of middle peptide bond
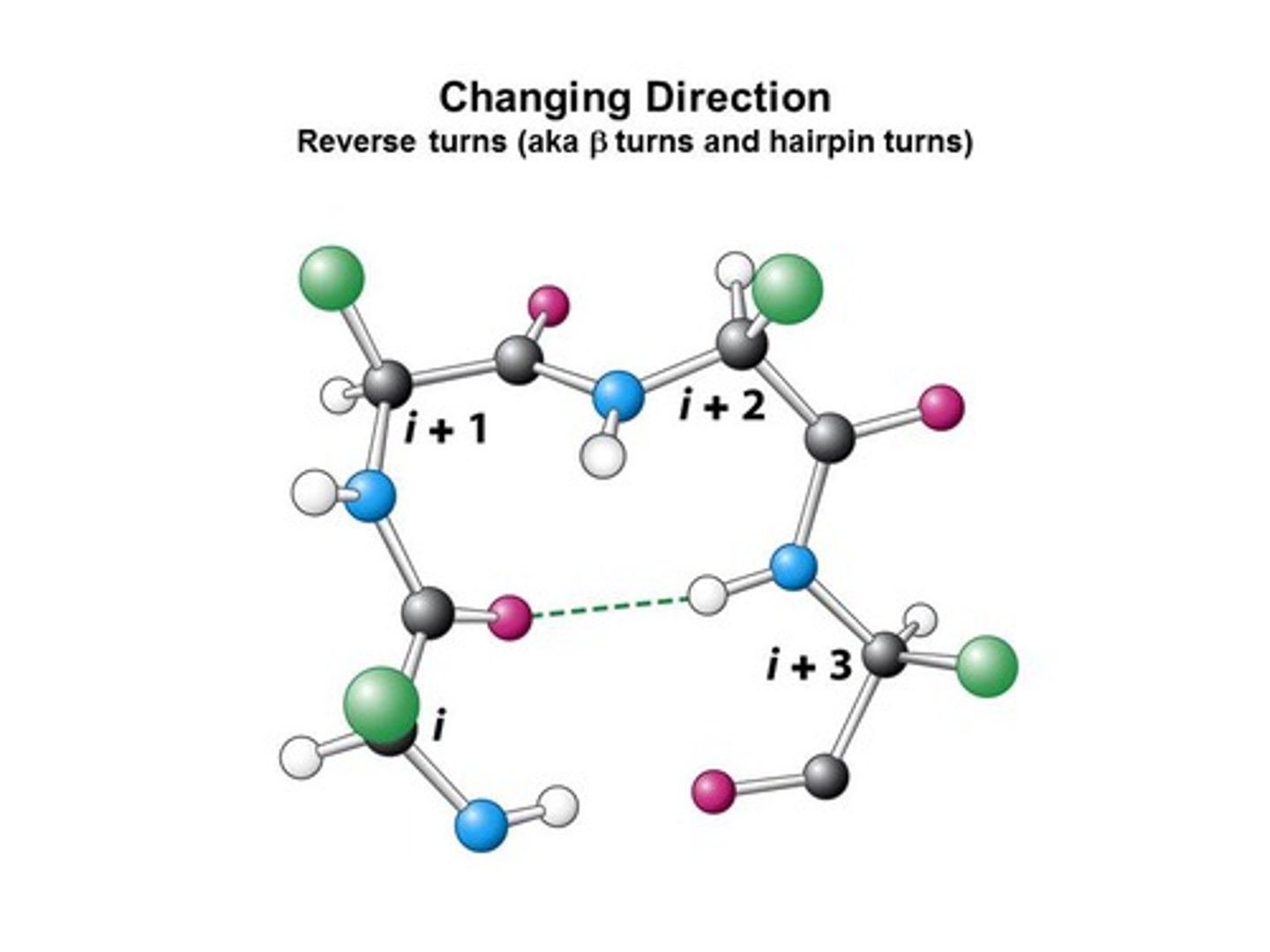
EXAMQ - what amino acids are prevalent in beta turns?
proline and glycine
the beta turn promotes ...
formation of antiparallel sheets.
three residue, zero pitch helix
EXAMQ - what is a random coil?
helices and strands are connected by loop regions of various length and shapes
is the random coil actually random and coiled?
no!! there is just no reoccurring bonding pattern, these loops are at the surface of the protein (full of hydrophilics!)
what do C=O and N-H bonds do in random coils?
they form H-bonds with water
where are random coils rich in?
charged and polar residues because they are exposed to the cytosol
random coils are often conserved. T/F
false
random coils are flexible and participate in binding events. T/F
true
what do amino acids prefer?
they exhibit only a preference for particular types of secondary structures; 50-80% accuracy.
beta turn - prefer proline and glycine!!
alpha helix - likes everything
beta sheet- doesn't prefer charged as much
what is a beta-hairpin structure?
two adjacent antiparallel strands joined by a 2-5 residue loop

what is a beta-alpha-beta motif?
two adjacent parallel beta strands connected by an alpha helix that is commonly positioned above the beta plane.
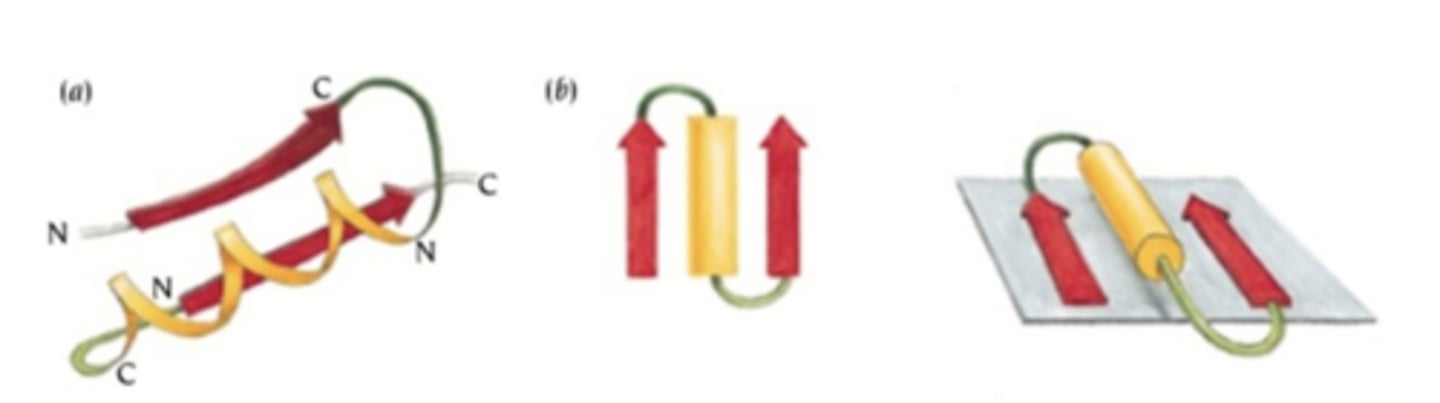
what is a coiled coil?
two helices wound around each other in a supercoil or super-helix => in your DNA (supercoil) which is good for packing
what reduces the number of residues per turn to 3.5 in super secondary structures?
the left-handed twist of the structure
after how many residues do the side chains repeat in supersecondary structures?
7 residues
what is alpha-keratin?
a fibrous protein found in hair, fingernails, claws, horns, and beaks
what does the sequence look like for alpha keratin?
the sequence consists of 311-314 residue alpha helical rod segments capped with non-helical N and C termini
the primary structure of helical rods in alpha keratin consists of....
7 residue repeats: (a-b-c-d-e-f-g)n where a and d are nonpolar
the alpha keratin structure promotes....
association of helices to form coiled coils
what is a tertiary structure?
secondary and supersecondary structure combined to make domains (<250)
what is a domain?
a polypeptide chain or part of a polypeptide chain that can fold into a stable tertiary structure in aqueous solution.
tertiary structures can consist of a single portion of the protein sequence. T/F
true
example of a tertiary structure
acetyltransferase
domains can combine to make ____________ proteins
multi-domain
often larger proteins don't contain larger domain, just _______ domains
more
proteins consisting of multiple domains evolved from
fusion of genes that once coded for separate proteins
90% of domains ________ in different proteins
re-occur
structural classification of proteins (SCOP)
database recognized five overarching class
SCOP is based on levels that embody the __________ among known proteins (manual curation)
evolutionary and structural relationships
CATH
class, architecture, topology, homologous superfamily
how is CATH different from SCOP?
CATH combines manual analysis with quantitative algorithmic analysis
what are the different classifications schemes for proteins?
class, fold, superfamily, and family
class
determined from overall composition of secondary structure elements in a domain
fold
describes the number, arrangement, and connections of these secondary structure elements.
family
includes domains with closely related amino acid sequences
what are the four major classes of protein structure?
all alpha, all beta, alpha/beta proteins (mixed), alpha+beta proteins (distinct)
protein quaternary structure
genetic economy and efficiency. creation of catalytic and regulatory sites at subunit interfaces. cooperativity.
cooperativity (quaternary structure)
between multiple catalytic sites via CONFORMATIONAL changes
EXAMQ - why was Anfinsen's experiment so important?
showed hat he could re-fold a protein using only the protein. all of the info necessary for folding the peptide chain into its 'native' structure is contained in the primary amino acid structure of the peptide.
how can ribonuclease be unfolded?
by treatment with urea and beta-Mercaptoethanol (MCE)
why do proteins fold?
to bury hydrophobic residues and expose polar residues. exposed polar residues interact w solvent waters
in globular proteins, stabilizing folding maximizes the .....
# of weak interactions (minimum free energy)
folding protein processed can be pictured as....
funnel of free energy
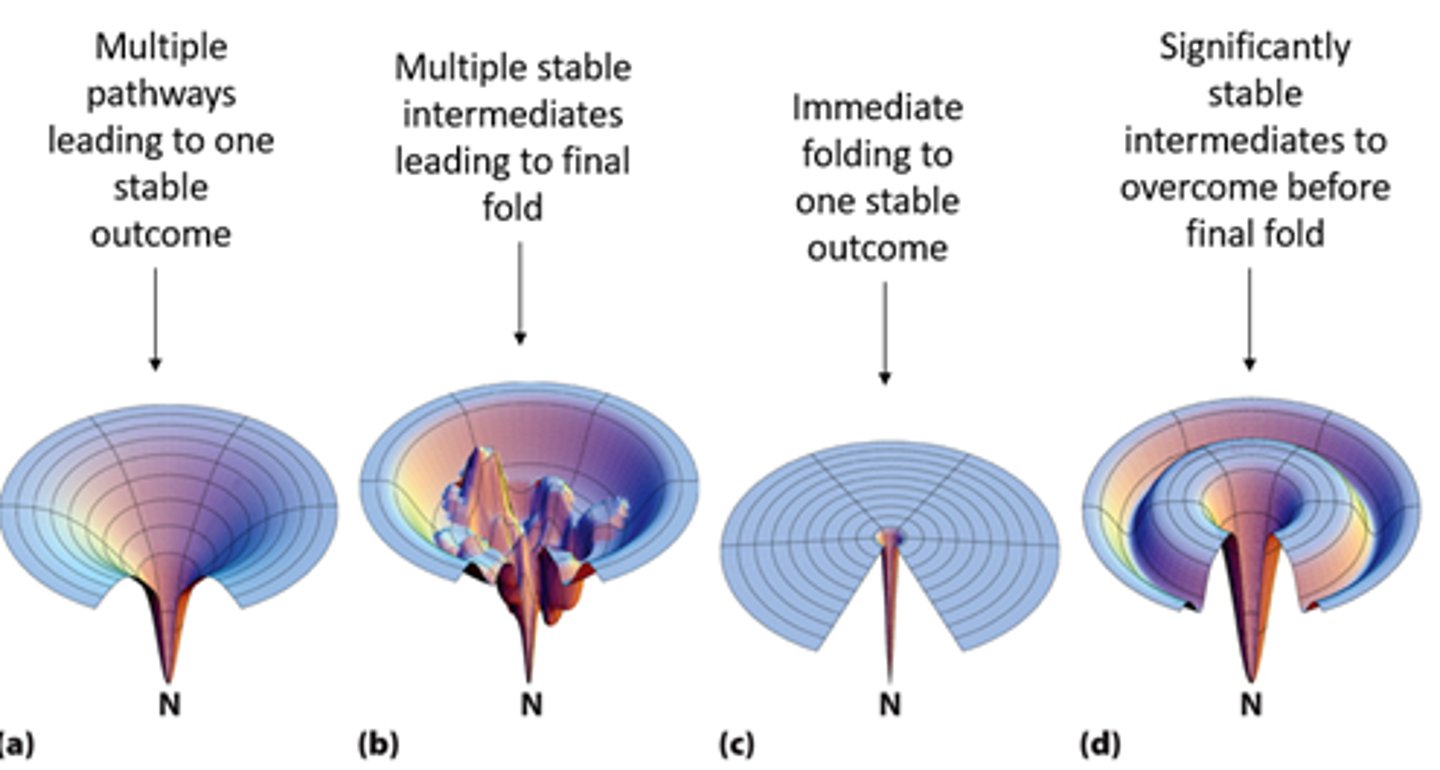
what does the rim of the funnel of free energy represent?
represents many unfolded states
what do polypeptides do in the funnel of free energy?
fall down the wall of the funnel to ever fewer possibilities and lower energies as they fold
funnel of free energy must have...
a favorable free energy - proteins are NOT static
proteins are __________ and __________
marginally stable, dynamic
what is the ∆G for protein folding?
-20 to -40 kJ/mol (small)
microcalorimetry of protein unfolding indicates
a favorable enthalpy change is the principal contributor to folding
what are some diseases of protein folding?
alzheimers, cystic fibrosis, cancer, hereditary emphysema, etc.
what protein is affected in alzheimers disease?
beta amyloid. misfolded beta amyloid accumulates in neural tissue forming deposits known as neuritic plaques
what protein is affected in cancer?
p53. this prevents cells with damaged DNA from dividing. one class of p53 leads to misfolding; the misfolded protein is unstable and then destroyed.
protein denaturation is
the loss of protein structure and function.
ex. protein of egg whites denatured during cooking
EXAMQ - melting temperature or Tm is the temperature at which _____________ of the protein is unfolded.
50%
what do chaperones do?
help proteins fold by preventing inappropriate liaions in the crowded cellular environment. it is found in all cells.
what do chaperones work to prevent?
intermolecular nonproductive interactions between hydrophobic sequences until productive folding interactions can occur.
how do chaperones drive folding?
through hydrolysis of ATP
what do chaperones recognize?
exposed hydrophobic residues
heat-shock proteins
upregulated when cells are exposed to heat
what are the principal chaperones?
Hsp70, Hsp60 (the chaperonins), and Hsp90
nascet proteins emerging from the ribosomes are met by...
ribosome-associated chaperones that can pass unfolded proteins to Hsp70
what are the different folding pathways for chaperones?
1. chaperone independent
2. Hsp70 assisted protein folding
3. Folding assisted by Hsp70 and chaperonin complexes
in bacteria, what is Hsp70 called?
DnaK
Hsp is involved with two domains what are they?
ATP binding domain and a domain that binds polypeptides with exposed hydrophobic regions
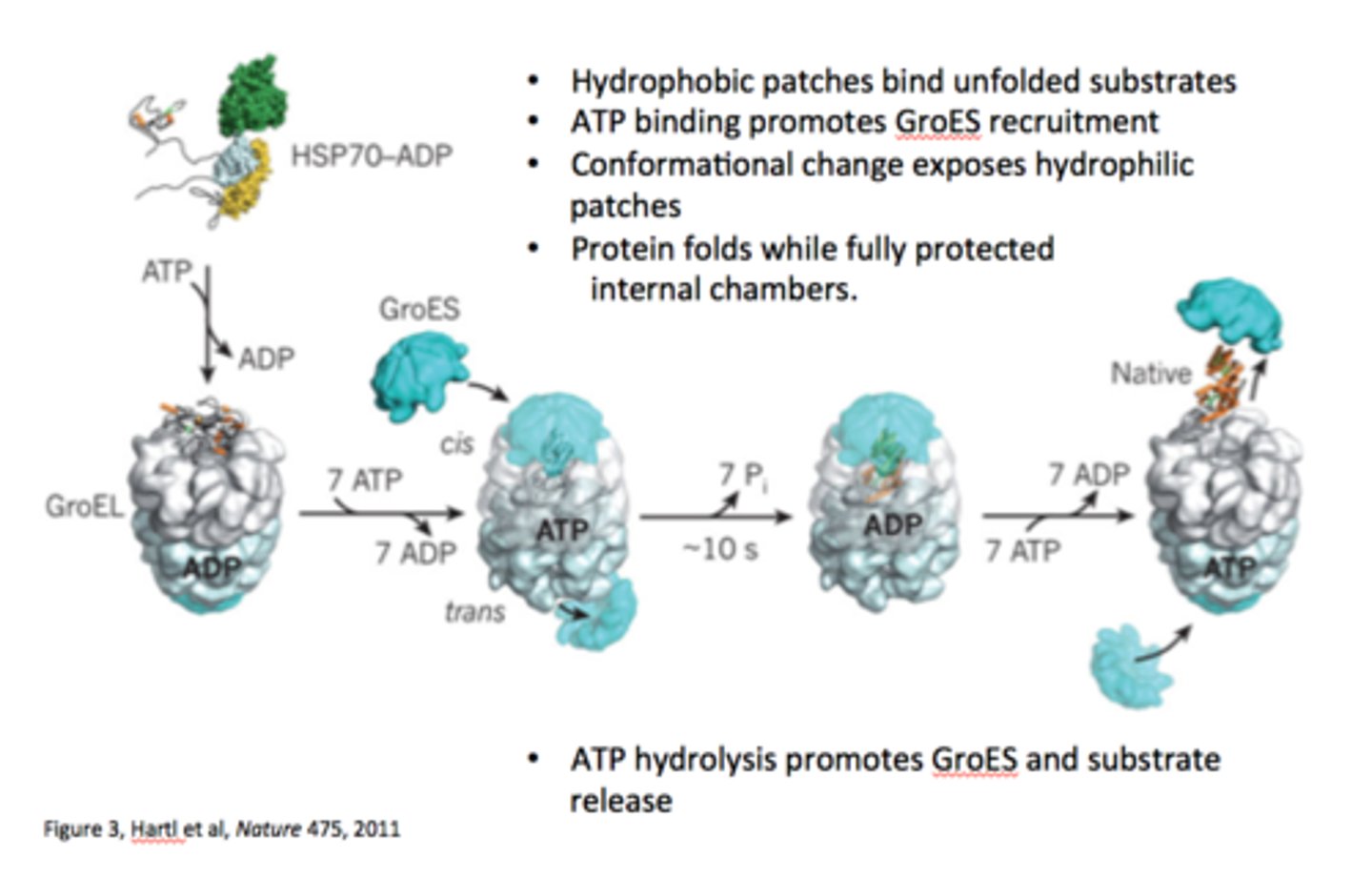
Hsp60 (GroES-GroEL complex in E.coli)
sequester partially folded proteins in an enclosed spaced - AKA "Anfensen cage"
substrate polypeptides in Hsp60 undergo ____________________________ that bury hydrophobic residues to produce the native form
forced unfolding followed by folding processes
what drives the Hsp60 complex?
ATP
in Hsp60, how are the chaperonins formed?
formed from two stacked rings of subunits.
GroES-GroEL structure
an unfolded or partially folded polypeptide binds to hydrophobic patches on the apical ring of alpha7 subunits, followed by ATP binding, forced protein unfolding, and GroES association.