chemical changes
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
exothermic reaction
An exothermic reaction is one that transfers energy to the surroundings so the temperature of the surroundings increases.
examples of exothermic reaction
Exothermic reactions include combustion, many oxidation reactions and neutralisation.
Everyday uses of exothermic reactions include self-heating cans and hand warmers.
An endothermic reaction
An endothermic reaction is one that takes in energy from the surroundings so the temperature of the surroundings decreases.
examples of endothermic
Endothermic reactions include thermal decompositions and the reaction of citric acid and sodium hydrogencarbonate. Some sports injury packs are based on endothermic reactions.
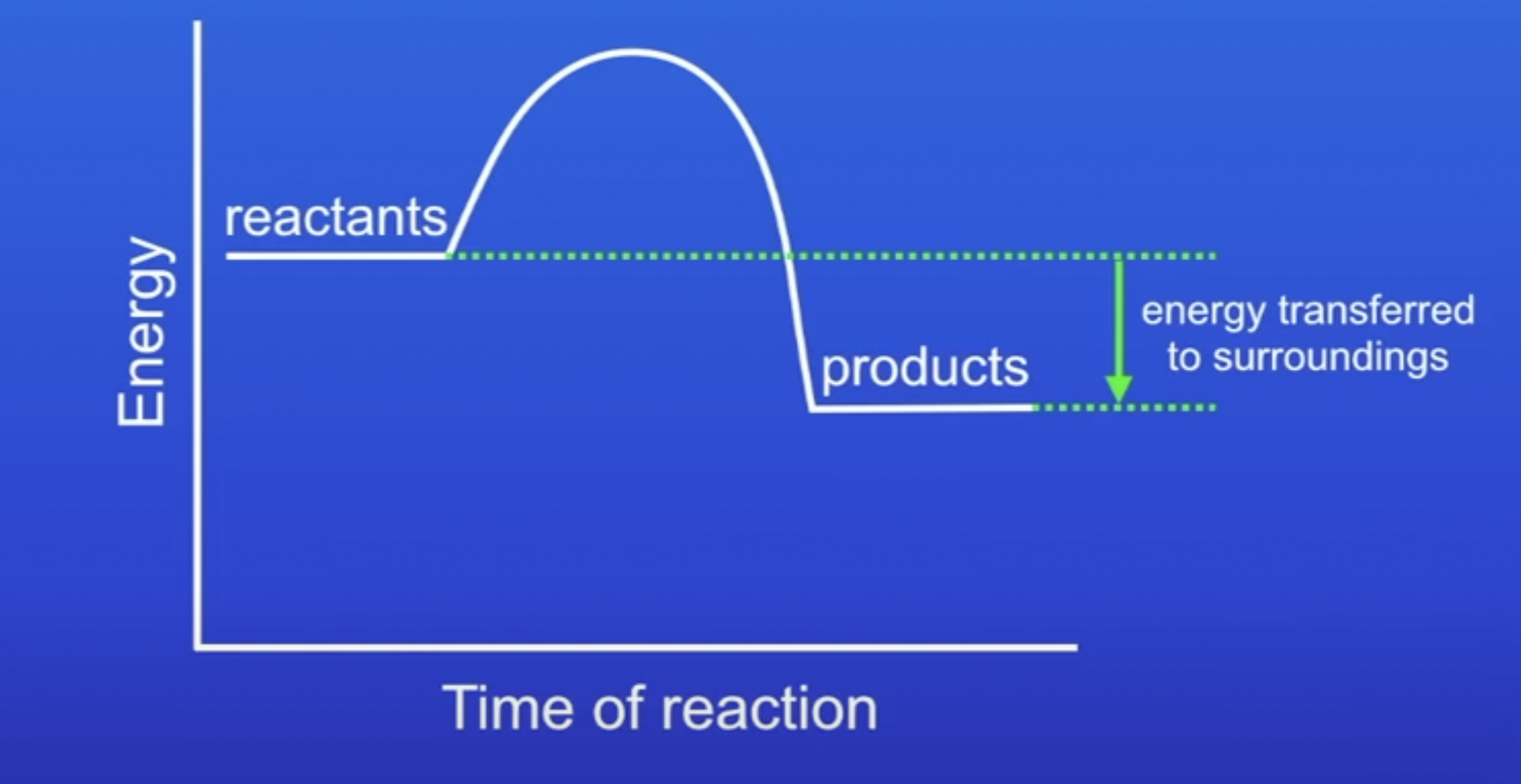
energy profile of exothermic reaction
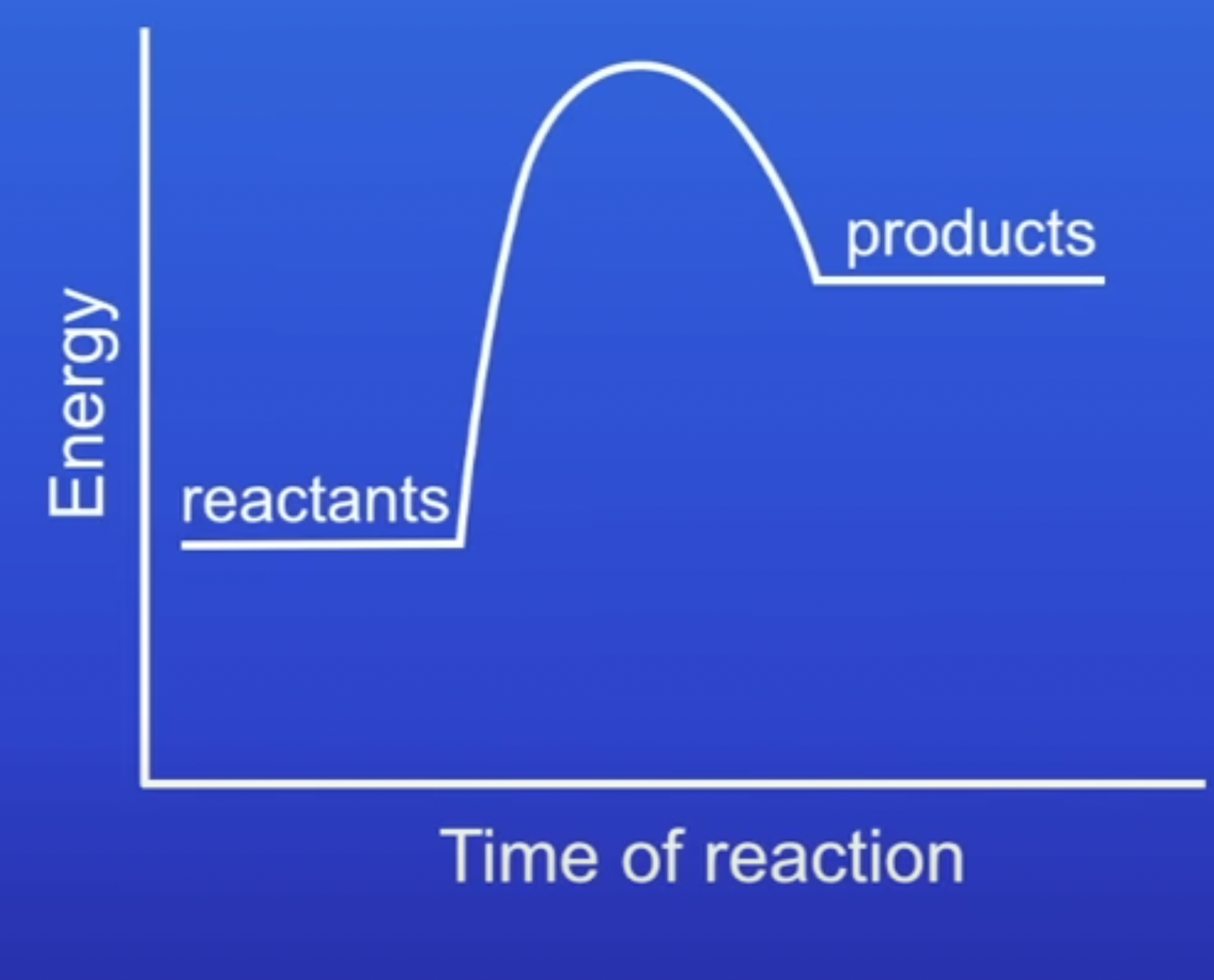
energy profile of endothermic reaction
energy in chemical reactions
Energy is conserved in chemical reactions. The amount of energy in the universe at the end of a chemical reaction is the same as before the reaction takes place. If a reaction transfers energy to the surroundings the product molecules must have less energy than the reactants, by the amount transferred.
activation energy
minimum amount of energy that particles must have in order to react is called the activation energy
-in an energy profile diagram, is from the reactants to the peak of the curve
how are energy changes shown in
-exothermic reaction
-endothermic reaction
shown as negative, because energy has been transferred from the chemicals
-showwn as positive because energy has been gained
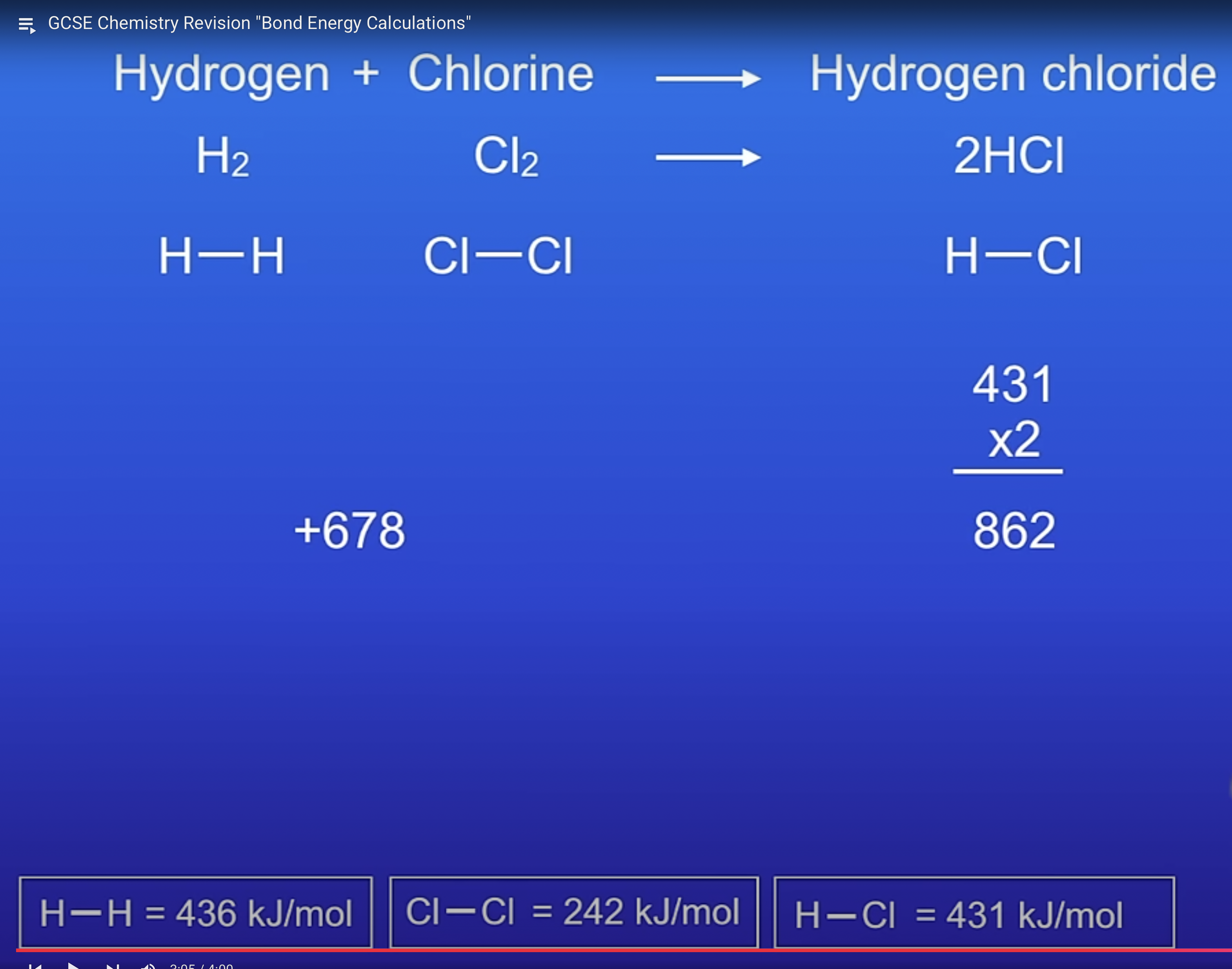
bond energy
-when we break a chemical bond, this requires energy (endothermic)
-making bonds releases energy (exothermic)
-every chemical bond has an energy value
-this tells us the energy required to break that bond

calculate the energy change
nswee= -184 kJ
Required Practical 4: Temperature Changes"
-investigate the temp change in an exothermic reaction (neutralisation reaction between Hal and alkali sodium hydroxide)
-we are going to add increasing volumes of sodium hydroxide solution to HCl and in each experiment we will measure the max temp reached
independent variable=volume of sodium hydroxide solution
dependent variable=max temp reached
control variable= volume and conc of HCl and conc of sodium hydroxide solution
start by using a measuring cylinder to measure 30cm3 of dilute HCl and transfer the acid into a polystyrene cup and we stand it inside a beaker which will stop it from falling over
we use a thermometer to measure the temperature of the acid
Use a measuring cylinder to measure 5cm3 of sodium hydroxide solution and we transfer this to the polystyrene cup
This point we fit a plastic lid to the cup and place the thermometer through the hole in the lid and use it to gently stir the solution_ because this reaction is exothermic it releases energy meaning temp rise and when the reading of the thermometer stops changing we record the highest temperature reached
We now rinse out and dry the polystyrene cup
Repeat the whole experiment using 10cm3 of sodium hydroxide solution and we then carry out the experiment several more times and each time we increase the volume of sodium hydroxide solution by 5cm3 until we reach a max of 40cm3 of sodium hydroxide solution. At this point, we repeat the whole experiment one more time so that we have two sets of results and now use these to calculate a mean value for the max temp reached for each volume of sodium hydroxide solution
At the end can plot a graph- as we increasing volume the max temp reached increases because when we add more particles of sodium hydroxide, they react with HCl and so more energy released but at a certain volume, the max temp starts to decrease because we r adding so much sodium hydroxide that there is not enough hcl meaning some of sodium hydroxide is unable to react. So because of this, the amount of energy released by the reaction has reached a max. The temp starts to decrease because we adding a greater volume of solution so energy released is now spread out into a greater volum- because of this when we add large volumes of sodium hydroxide solution and so the energy released is now spread out into a greater volume
In experiment why do we use a polystyrene cup with a lid
In the experiment we are measuring the temp and so we want to reduce any heat losses. Polystyrene is a good thermal insulator so this reduces heat loss. The lid reduces heat loss to the air
What can we do if we have two metals and place them in an electrolyte
We can produce electricity
Electrolyte
A simply solution that can conduct electricity
cell( two metals in an elecrolyte)
A cell can only produce electricity for a certain period of time. Eventually, the chemicals in the cell run out and the reaction stops. Cells will only produce electricity if we use metals with different reactivity. The bigger the reactivity between the two metals the bigger the pd produced by the cell. The electrolyte also affects the pd
A battery
Contains two or more cells connected in series to produce a greater voltage
non-rechargeable batteries
In alkaline batteries, at some point the reactants in the batteries run out and no more electricity is produced and there is no way that we can reverse these reactions.
Rechargeable batteries
They can be recharged because we can reverse the chemical reactions when we apply an electrical current
Cells and batteries
Use an chemical reaction to produce electricity and eventually the reactants are used up and the electricity stops
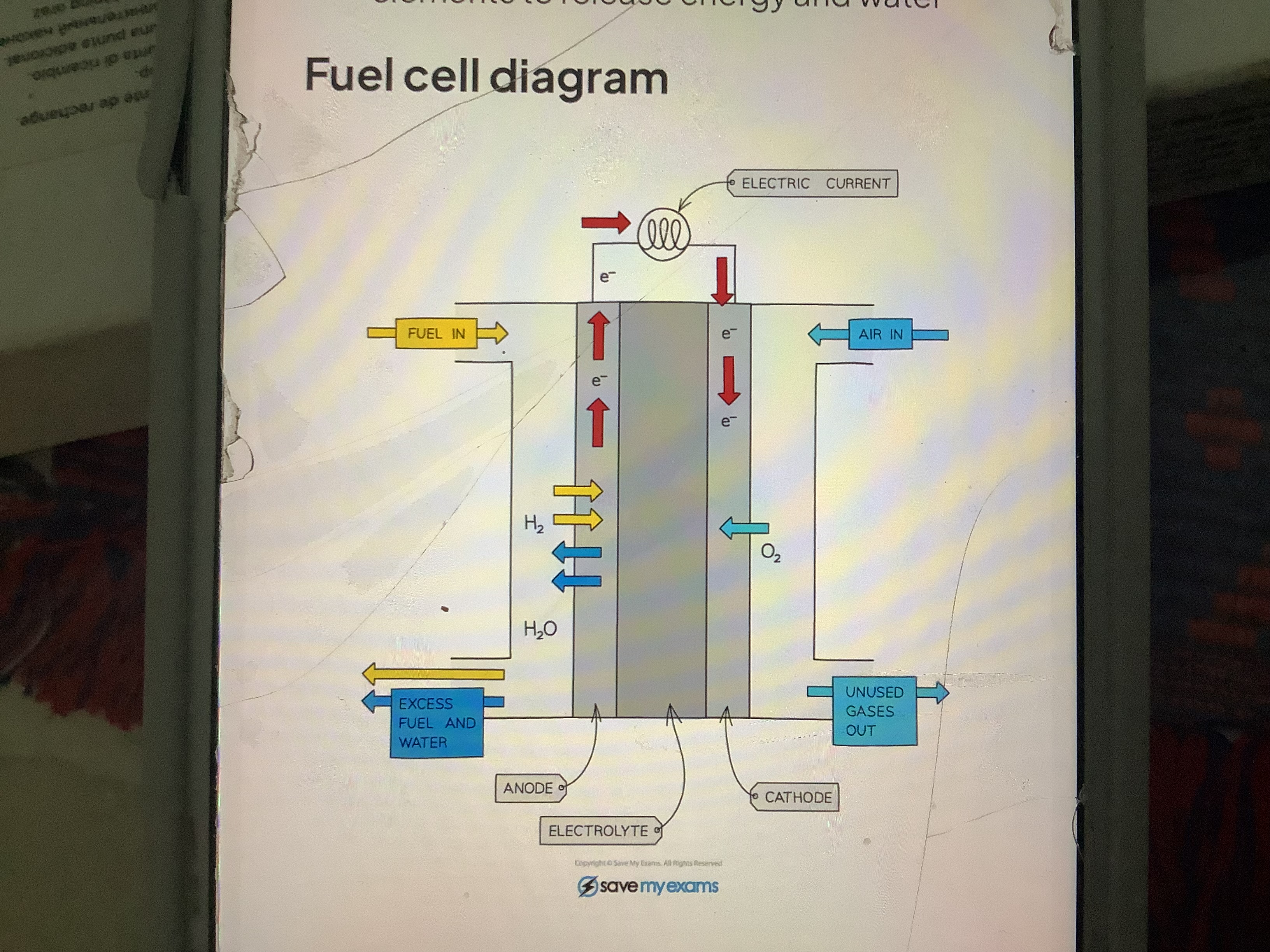
How does a hydrogen fuel cell work
is an electrochemical cell in which a fuel donates electrons at one electrode and oxygen gains electrons at other electrode.
These cells are becoming more common in the automotive industry to replace petrol or diesel engines
As the fuel enters the cell it becomes oxidised which sets up a pd within cell
Different electrolytes and fuels can be used to set up different types of fuel cells
Hydrogen oxygen fuel cell combines both elements to release energy and water
What happens to the polarity on the electrodes in fuel cells
The polarity on the electrodes are other way around- anode is negative and cathode is positive
Advantages of hydrogen fuel cell
-do not produce any pollution
produce more energy per kg than either petrol or diesel
No power is lost in transmission as there are no moving parts unlike an internal combustion engine
No batteries to dispose of which is better for the environment
Continuous process and will keep producing energy as long as fuel is supplied
disadvantages of hydrogen fuel cells
-materials used in producing fuel cells are expensive
-high pressure tanks are needed to store the oxygen and hydrogen in sufficient amounts which are dangerous and difficult to handle
fuel cells