Biochem SI 7- Enzymes III and Chymotrypsin
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Match the following abbreviations with their proper meaning. Then define each term in common words.
a. Vmax Turnover number
b. KM Reaction rate at specific substrate concentration
c. kcat Maximum reaction rate
d. V0 Substrate concentration
e. [S] Michaelis constant
a. Vmax→ Maximum reaction rate: The fastest possible rate of the reaction when the enzyme is completely saturated with substrate
b. Km→ Michaelis constant: The substrate concentration at which the reaction rate is half of Vmax, a measures how strongly the enzyme binds to its substrate. A lower KM means the enzyme binds the substrate more tightly
c. kcat→ Turnover number: the number of substrate molecules an enzyme can turn into product per second when the enzyme is fully saturated, basically, how many "jobs" each enzyme molecule can finish per second
d. V0→ Reaction rate at specific substrate concentration: essentially the "starting speed" of the reaction
e. [S]→ Substrate concentration: How much substrate is present
Draw a sketch of a Michaelis-Menten graph and a Lineweaver-Burk plot on the board. Be sure to label Vmax, KM, the x and y axis, and the slope (where applicable).
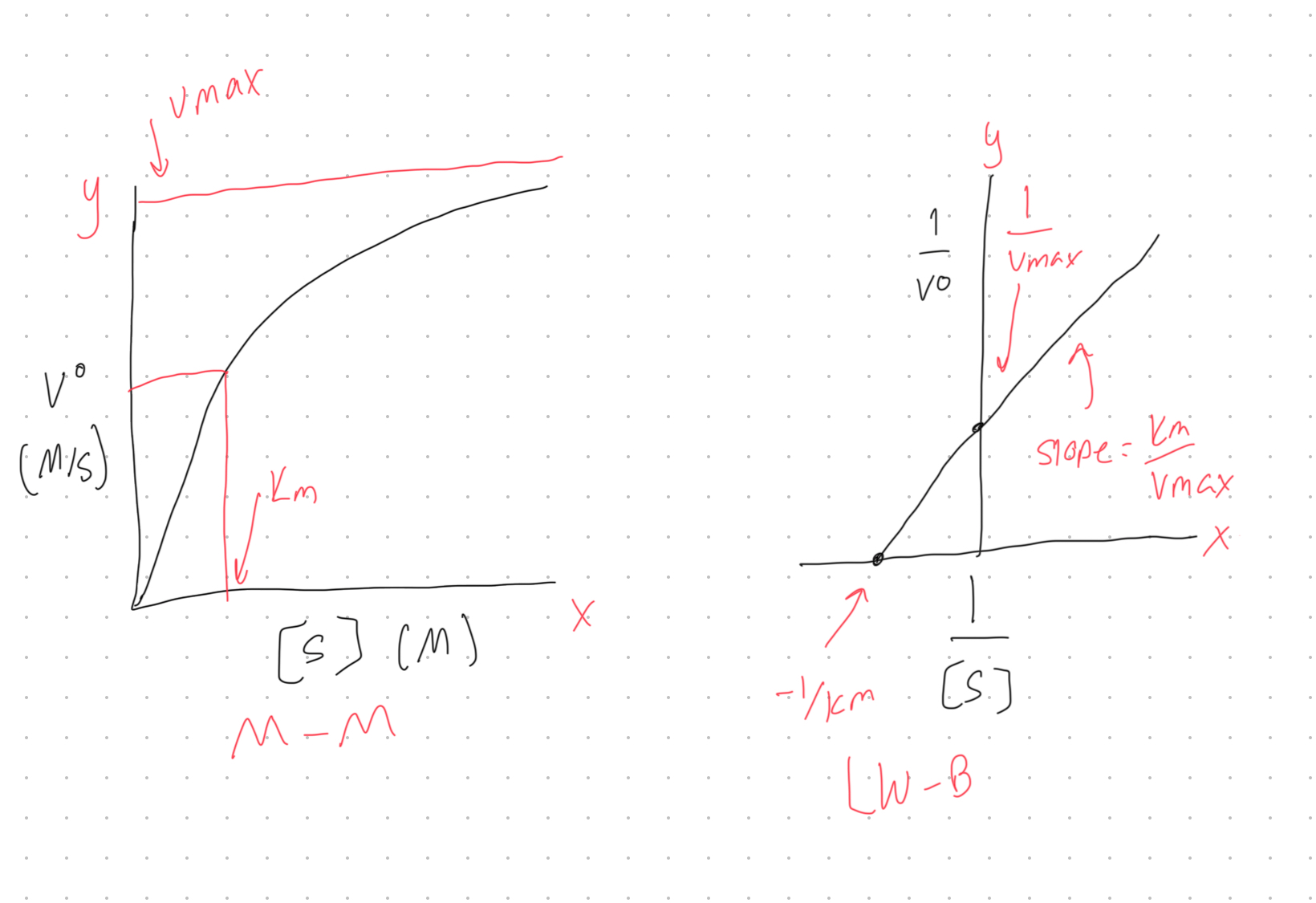
Describe what the michaelis constant measures.
Michaelis constant (Km) measures the affinity of an enzyme for its substrate→ [S] at ½ Vmax.
Low Km→ high affinity/high efficiency at low [S]
High Km→ low affinity/low efficiency at low [S]
Describe what the turnover number measures.
Turnover number (kcat)→ the number of P molecules produced per enzyme per unit of time when enzyme is fully saturated with substrate.
Describe what kcat/KM measures
kcat/Km (specificity constant) measures the catalytic efficiency of the enzyme
Higher specificity constant→ more efficient enzyme
Lower specificity constant→ less efficient enzyme
This ratio can be used to compare an enzyme’s preference/specificity for different substrates.
What does reversible enzyme inhibition entail? What about irreversible?
Irreversible enzyme inhibitors→ bind covalently or noncovalently to the enzyme, but with a negligible dissociation constant
Reversible inhibition→ characterized by a rapid dissociation of the enzyme-inhibitor complex
Describe each of the following models of inhibition and how they affect kinetics: competitive
Competitive inhibitors bind at the active site of the enzyme and compete directly with the enzyme’s natural substrate. Competitive inhibitors increase Km and do not affect Vmax. This is because they reduce the number of active ES complexes that produce product, increasing the amount of substrate needed to reach ½ Vmax. Vmax is not impacted because active ES complexes will still produce P at the same rate.
Describe each of the following models of inhibition and how they affect kinetics: noncompetitive
Noncompetitive inhibitors bind at an allosteric site and do not affect the active site. They decrease Vmax but do not affect Km. They can form unproductive EI or ESI complexes and do not impact the affinity of the enzyme for the substrate, rather they decrease the rate at which product is formed. Vmax decreases because, even when all enzyme is saturated with substrate, unproductive ESI complexes cause the maximum rate to decrease.
Describe each of the following models of inhibition and how they affect kinetics: uncompetitive
Uncompetitive inhibitors bind only to the ES complex near the active site, forming an unproductive ESI complex. They cause both Km and Vmax to decrease. Km decreases because the inhibitor can “lock” the substrate in place and prevent it from being converted to product, causing the binding affinity to appear higher. Vmax decreases because, even when all enzyme is saturated with substrate, unproductive ESI complexes cause the maximum rate to decrease.
Describe the inhibitor in competitive inhibition. What does it look like?
In competitive inhibition, the inhibitor often mimics the enzyme’s natural substrate. More potent inhibitors can also mimic the transition state, as enzymes bind it more tightly than its natural substrate to increase reaction favorability→ transition-state analogs.
What type of enzyme is chymotrypsin, and where is it produced in the body?
It is a serine protease that is produced in the pancreas and secreted into the small intestine.
What three amino acids make up the catalytic triad in chymotrypsin?
Serine 195
Histidine 57
Aspartate 102
Which amino acid in the catalytic triad acts as a nucleophile?
Serine 195→ its deprotonated hydroxyl attacks the peptide carbonyl carbon
What role does Asp102 play in the catalytic triad?
Asp 102 is negatively charged, so it is able to stabilize the protonated form of His 57. It stabilizes His 57 through H-bond interactions, which increases its basicity (higher pKa than normal ~7.5) and its ability to deprotonate Ser 195.
What type of R-groups does the S₁ pocket prefer to bind?
Large/bulky/hydrophobic/aromatic R-groups→ Trp, Tyr, Phe, Met
What is the purpose of the oxyanion hole in the active site?
The oxyanion hole stabilizes the tetrahedral intermediate formed in the chymotrypsin reaction via H-bonds
What are the two main steps in the chymotrypsin mechanism (overall reaction phases)?
Acyl enzyme intermediate formation→ peptide bond is cleaved and amine component leaves
Hydrolysis of acyl enzyme intermediate (deacylation)→ water hydrolyzes intermediate and carboxylic acid component leaves
What is the name of the covalently bound enzyme intermediate formed after peptide bond cleavage?
Acyl enzyme intermediate
Predict what would happen to enzyme activity if His57 were mutated to Ala. Explain why.
If His 57 were mutated to Ala, enzyme activity would lower drastically or stop entirely. This is because His 57 is required to deprotonate Ser 195 so it can act as a nucleophile and begin the reaction. Alanine’s side chain consists only of a nonpolar methyl, so it would be unable to deprotonate Ser 195, which is necessary for the reaction to occur.
A researcher observes that replacing the hydrophobic residues in the S₁ pocket with polar residues drastically reduces chymotrypsin activity. Explain the molecular basis for this result.
The hydrophobic interactions that occur in the S1 pocket are necessary for binding the P1 side chain of the substrate. This would cause a dramatic decrease in substrate affinity/specificity and catalysis.
Imagine designing a drug that mimics the transition state of the peptide cleavage reaction. How would this affect the rate of catalysis?
It would greatly reduce the rate of catalysis, as the drug would compete directly with the natural substrate. It would further decrease because enzymes tends to have greater specificity/affinity for the transition state than the natural substrate. This would cause the drug to act as a very potent inhibitor (transition-state analog), lowering the rate of catalysis.