Chapter 3 catecholamines
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
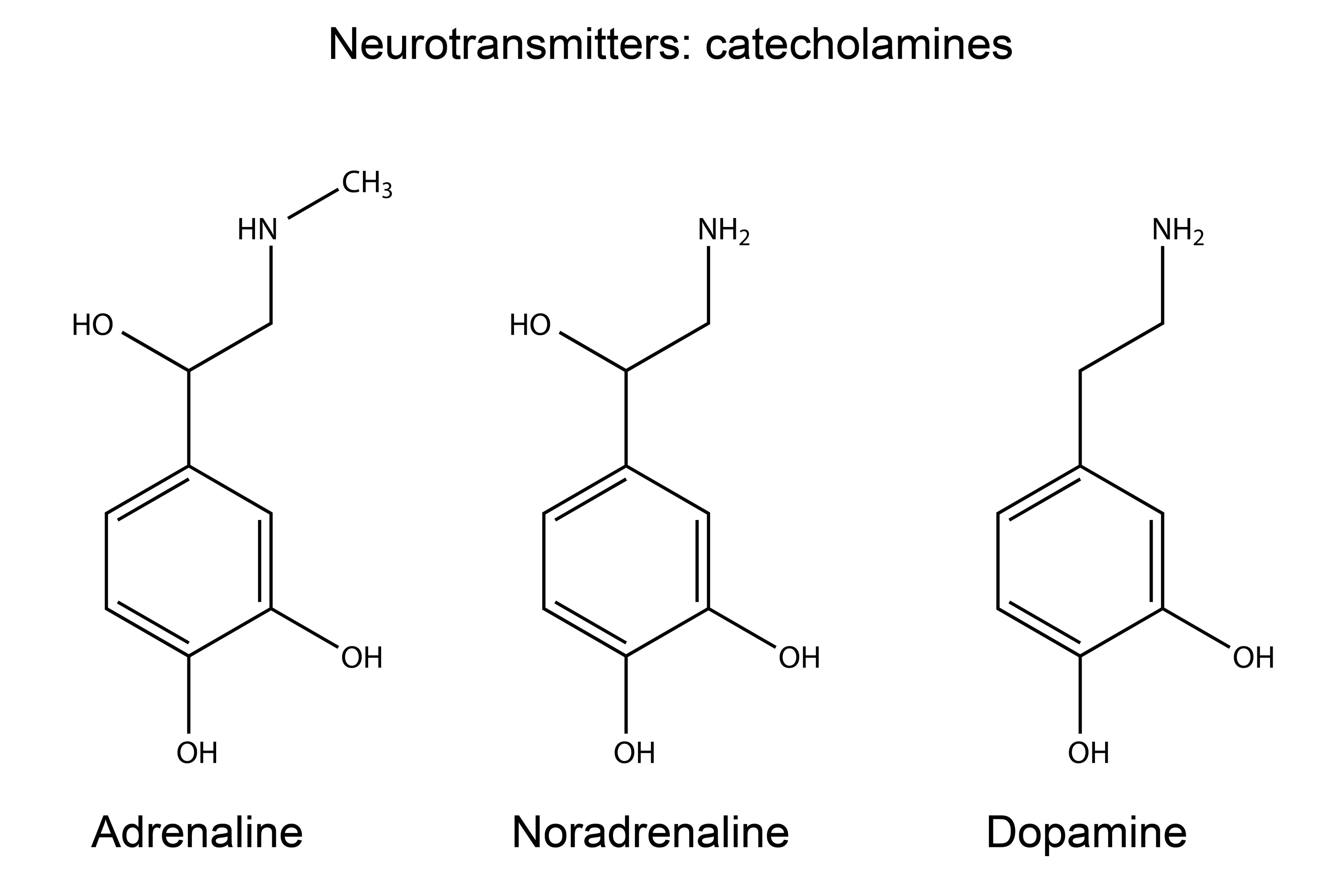
Catecholamines
Amino Acid Derived hormone type
• Have a catechol group and an amine group • Derived from tyrosine
• Dopamine, Norepinephrine, Epinephrine
• Synthesized in the Adrenal Medulla
• Primary function for NE and E • Responds to stress
Stimuli for catecholamine release
Stress Response is the stimuli
• Emotional Stress
• Fear/Anxiety • Excitement
Physical Stress
• Exercise, Pain/Injury • Hypotension/Hypovolemia
• Environmental Stress
• Cold/Heat • Hypoxia
• Metabolic Stress
• Hypoglycemia
Need gluocse in the blood in stressful situations through catecholamine release
Autonomic Nervous System
• Efferent Division of Nervous System
• Not under Voluntary Control
• Sympathetic Branch
• Fight or Flight • Thoracolumbar neurons(middle of spinal cord)
Parasympathetic Branch
• Rest & Digest • Cervical and Sacral neurons(start and end of spinal cord
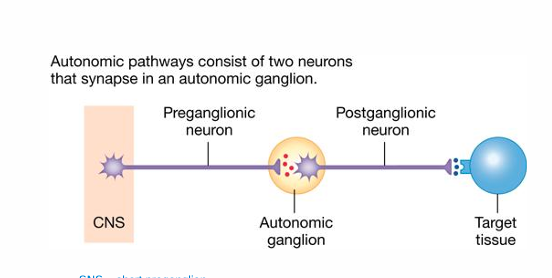
Ganglionic Pathways
ANS uses a two-neuron pathway
Preganglionic Neuron & Postganglionic Neuron
Divergence
• One preganglionic neuron may synapse onto as many as 32 postganglionic neurons
SNS
• Short preganglionic, long post ganglionic
PNS
• Long preganglionic, short post ganglionic
Receptors & Neurotransmitters in the CNS for nervous sytems
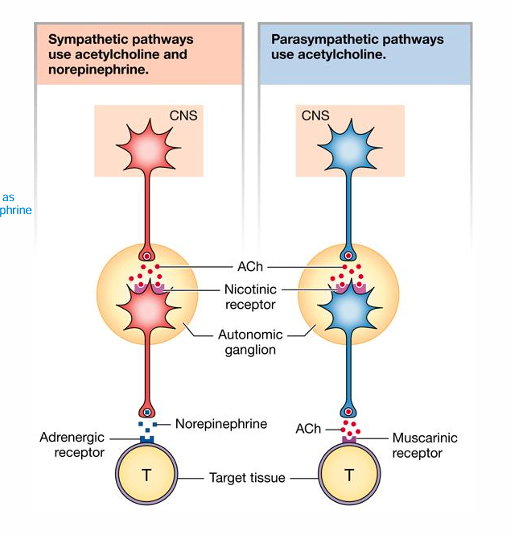
• Preganglionic onto Postganglionic
• Same for SNS & PNS • ACh onto a nicotinic receptor
Postganglionic onto Target
• SNS • Norepinephrine onto Adrenergic Receptor • α and β Adrenergic Receptors
• PNS • ACh onto Muscarinic receptor
Sympathetic pathways use acetylcholine and norepinephrine, parasympathetic pathways use acetylcholine only
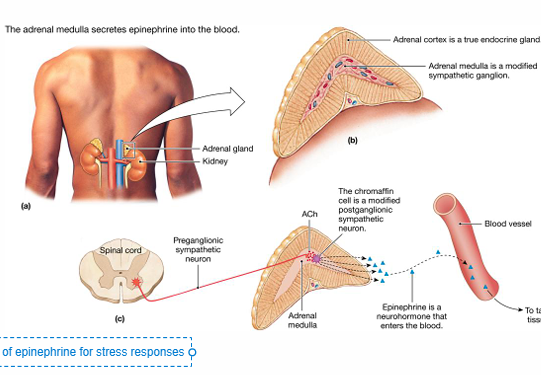
Adrenal medulla
• Modified Sympathetic Ganglion
SNS preganglionic neurons synapse onto this adrenal structure
• Stimulated in response to a significant stressor
Releases Epinephrine (80%) and NE (20%) into the blood •
Neurohormone • Systemic, less selective responses (any tissue with adrenergic receptors)
Neurons have lots of norepinephrine, but the blood requires a lot of epinephrine for stress response
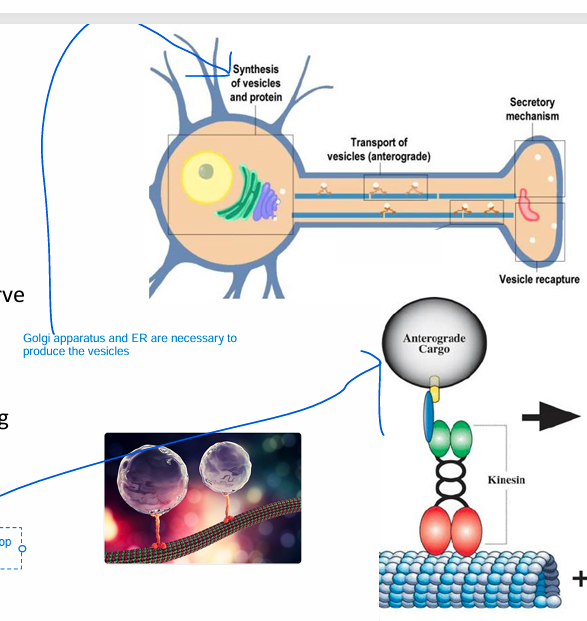
Synaptic ACh Storage and Transport
ACh is synthesized in cytosol (in the nerve terminal) and stored in vesicles
Golgi apparatus and ER are necessary to produce the complex vesicles
• Vesicles (empty) are formed in the cell body
• Transported to nerve terminal along microtubules via the motor protein Kinesin(Kinesin walk along the microtubules)
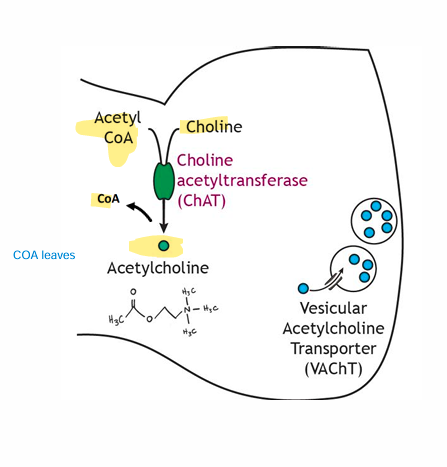
What is the enzyme responsible for ACh formation?
• Choline Acetyltransferase
• Located in cytosol of axon terminal
• Converts Choline + Acetyl CoA to Acetylcholine
• Choline brought in via membrane transportetransport
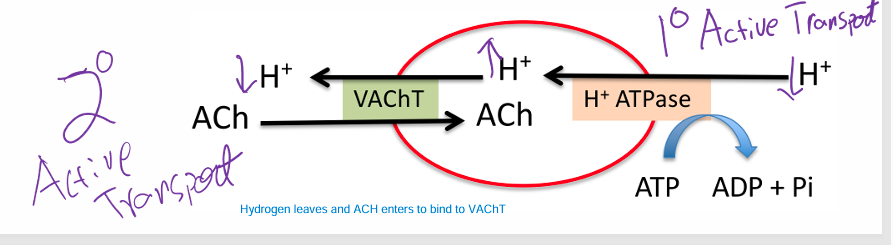
Synaptic ACh Entryways
• Vesicles contain two membrane proteins that allow entry of ACh
• H+ ATPase • Causes H+ to build up in the Vesicle to transport ACh as well( 1 degree = co-transporter)
• VAChT (Vesicle ACh Transporter) • Swaps H+ for AC(2 degree = antiporter)
ACh Release
Vesicles with ACh dock at the membrane, but do not release ACh. • SNARE binding vesicle/membrane
Calcium (from VG Calcium channels) binds to synaptotagmin.
Calcium binding to synaptotagmin causes displacement of complexin
This causes vesicle/membrane fusion and the release of vesicular contents (ACh)
Afterwards, vesicles are reabsorbed and recycled or degraded.
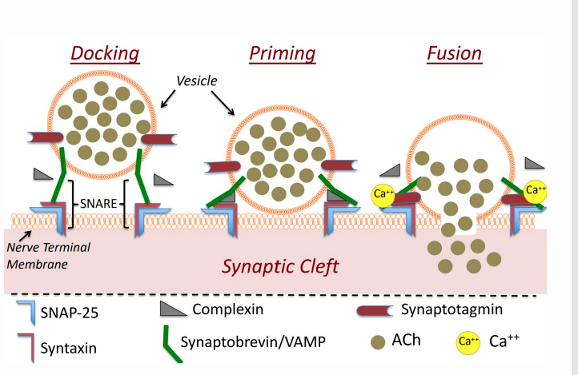
ACH binding and Recycling
ACh binds to target, opening sodium channels
Sodium influx opens Voltage-Dependent Calcium Channels (VDCC)
• Calcium influx into the chromaffin cell or nerve terminal of SNE postganglionic neuron
ACh in the synaptic cleft is hydrolyzed by Acetylcholinesterase to Choline and acetate
Choline is then brought back into the preganglionic neuron via a sodium cotransporter (using sodium gradient for energy)
acetate leaves the cell and gets metabolized by liver for excretion(no recycling)the
Norepinephrine in SNS Neurons(Tyrosine pathway)
• Tyrosine is brought in via an AA transporter
• Tyrosine->DOPA->Dopamine
• Dopamine enters a vesicle and is converted to NE and stored
• Enters via VMAT2 transport protein
• Action Potential opens VDCC in the postganglionic neuron • Calcium influx causes vesicle release of NE.
Norepephrine in SNS Neurons
NE activates adrenergic receptors (GPCRs) • α1, α2, β1, β2, β3
NE is recycled via the NE transporter (NETr)
NE induces negative feedback via α2 activation
Some NE diffuses to blood (metabolized by the liver)
ACH broken down by enzyme in membrane, NE is recycled by being received by a transporter and then being transported into the cell for recycling
Neuroendocrine Catecholamine Release diagram
chromaffin cells produce catecholamine when they are activated by Ach
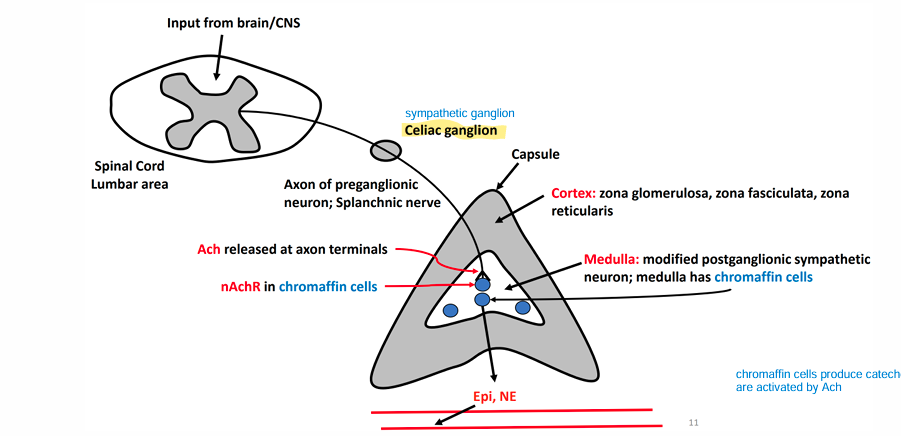
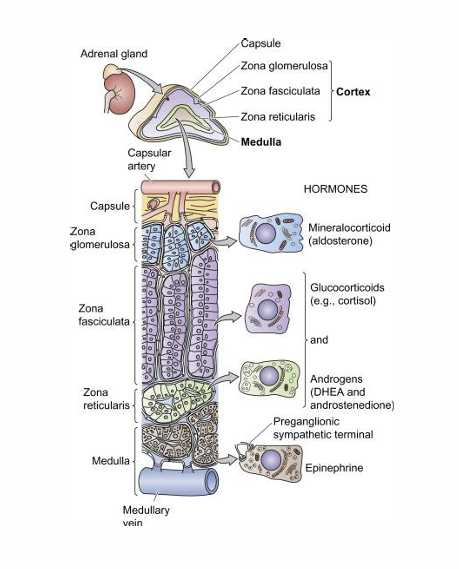
Adrenal Gland
• Superior to the kidneys
• Multilayered
• Different layers produce different hormones
• Cortex (True Endocrine Gland)
• Zona Glomerulosa(upper layer) • Zona fasciculata(middle thick layer) • Zona Reticularis(bottom layer)
• Medulla (Modified Sympathetic Ganglion)
• Chromaffin cells • Synthesize and secrete catecholamines
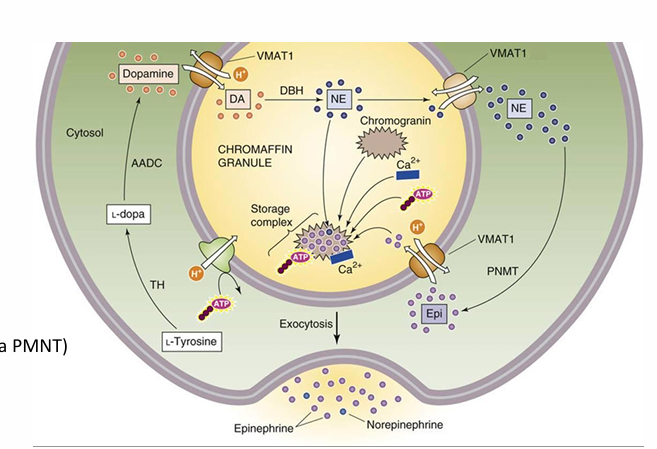
Epinepherine Synthesis
The synthesis for this catecholamine hormone uses VMAT transporters (catecholamine/H+ antiporter)
NE leaves the vesicle • Converted to epinepherine in the cytosol (via PMNT)
Epinepherine enters vesicle
Stored in a complex with chromogranin proteins.
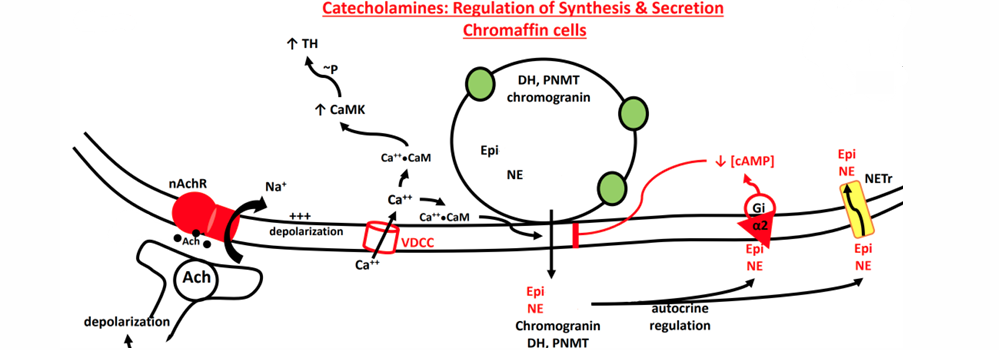
Catecholamine Release in Chromaffin Cells
SNS stimulation of chromaffin cells causes Na+ influx • ACh binding to nAChR
Na+ induced depolarization opens VDCC(voltage gated ion channel) • Initiates exocytosis
Similar to exocytosis of ACh from neurons
• Larger vesicles in chromaffin cells • Slower • Additional accessory proteins. • Stimulated by Calcium Calmodulin • Stimulates accessory proteins • Interacts with Synaptotagmin
Negative Feedback •
Catecholamines on α2 receptors • Reduces SNARE interaction, slowing exocytosis
• ATP (released from vesicles) • Closes VDCC by GPCR cascade
binds with CaM
• Facilitates exocytosis • Stimulates CaMK • Phosphorylates (activates) Tyrosine Hydroxylase • Leads to increased catecholamine synthesis
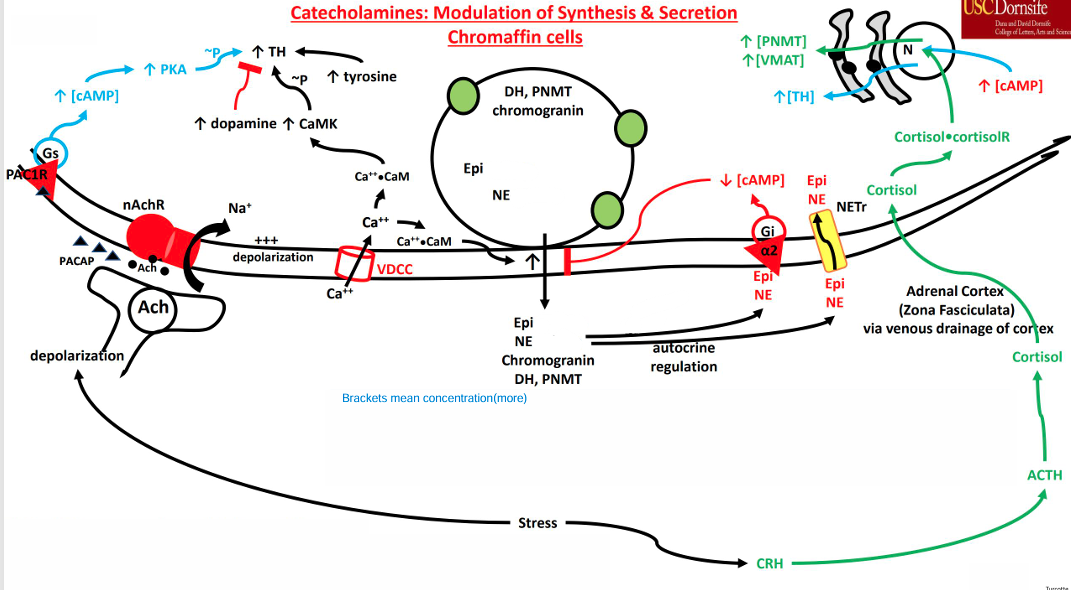
Effects of Cortisol
• Stress response causes cortisol to be released from the Zona fasciculata(middle layer of the adrenal cortex)
• Cortisol reaches the adrenal medulla
• Venous drainage
• Binds to nuclear receptors in chromaffin cells • Increases gene expression to induce synthesis of PNMT and VMAT( enzyme converter of norepinephrine to epinephrine and transporter of epinephrine respectively)
PACAP
Splanchnic Nerve also secretes Pituitary Adenylate Cyclase Activating Polypeptide (PACAP)
Binds to the PAC1R
• GPCR (Gs) • Leads to an increase in cAMP
• cAMP activates PKA • Phosphorylates and stimulates Tyrosine Hydroxylase • cAMP activates CREB (cAMP Response Element Binding Protein)
• Increases gene expression to induce synthesis of Tyrosine Hydroxylas
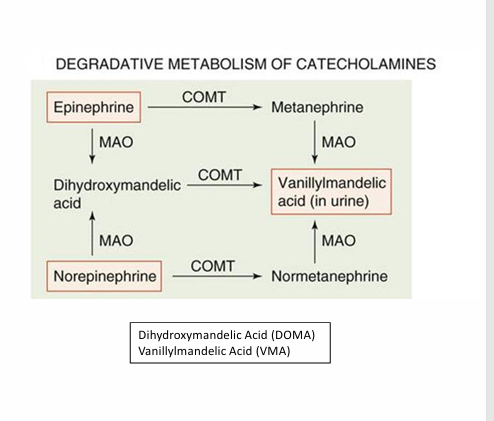
Catecholamine degredation
enzymes present in the liver are responsible for this catecholamine process
Catechol-O-methyltransferase (COMT): COMT makes dihydroxy mandelic acid into vanillylmandelic acid, norepinephrine to normetanephrine, and epinephrine to metanephrine.
Monoamine Oxidase (MOA): MOA takes catecholamines(Norepi and epi) and converts them to dihydroxy mandelic acid
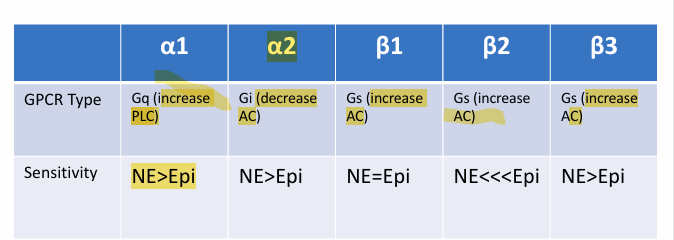
Adrenergic receptor
GPCRs
α1, α2, β1, β2, β3
Activated by NE released from SNS postganglionic neurons (Neurotransmitter)
Activated by circulated NE and Epi released from the adrenal medulla (Neurohormone)
varying locations, sensitivities, and effects
Beta 2 Favours epinephrine response due to the shape of the receptor.
All beta receptors and alpha 1 are Gs(adenylate cyclase activator) A2 is GI(inhibitors adenylate cyclase AC)
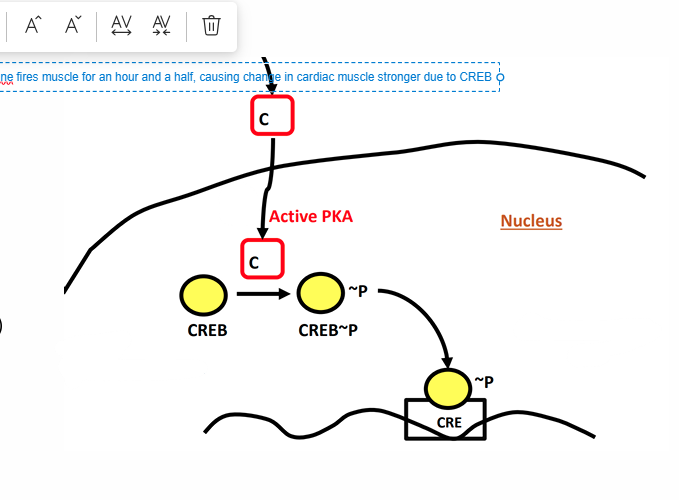
CREB(cAMP Response Element Binding Protein)
This protein is used to gain gene expression from GPCR
More of this protean means more stimulation on GPCR to release
• Enables gene transcription from a membrane-bound receptor
PKA activation can activate this protein (phosphorylates CREB at Serine 133)
• Chronic activation can lead to appreciable gene transcription(continuous activation)
Increased mitochondrial biogenesis • Cardiac muscle remodeling(more cardiac strength) • Uncoupling protein (UCP1) generation in brown adipose tissue receptor
Tyrosine hydroxylase
This enzyme is made in the process of norepinephrine creation
This enzyme is the tyrosine enzyme that makes Tyrosine to L-Dopa
More tyrosine hydroxylase means more L-Dopa for catecholamine production
α1 Adrenergic Receptors
Vasculature • Vasoconstriction
Eye • Dilator muscle contraction (pupil dilation)
GI tract • Sphincter Contraction
Genitourinary tract • Internal urinary sphincter contraction • Vas deferens and prostate smooth muscle (mediates ejaculation).
closes the urinary sphincter, stops the need to go to the bathroom, and also closes smooth muscles to stop the reproduction process
parasympathetic nervous system is responsible for arousal; a quick sympathetic nervous system response mediates arousal
Salivary Glands • Reduces saliva production
Liver • Promotes glycogenolysis and gluconeogenesis • Inhibits glycogen synthesis
Glycogenolysis: glycogen > glucose, gluconeogenesis: glucose from carbohydrates(want more glucose during stress)
• Pancreas • Inhibit insulin secretion
α2 Adrenergic Receptors
Skin and Gut Blood Vessels • Vasoconstriction(want extra vasoconstriction in skin and gut, that’s why alpha has this effect)
Sympathetic Neurons and Adrenal Medulla • Negative Feedback
Pancreas • Inhibit Insulin Secretion • Stimulate Glucagon Secretion(glucagon encourages more glucose in blood)
GI Tract Neurons • Reduce GI motility
Platelets • Blood Clotting
a constrictor 2 the death
β1 Adrenergic Receptors
Heart • Increase Heart Rate • Increase Contractility(most important regulated area)
Kidneys • Stimulate production of hormones (RAAS Pathway hormones) • Increase blood pressure • Decrease urine production • Increase vasoconstriction(just know RAAS pathway increases blood pressure, no need to know all hormones)
Adipose • Stimulate lipolysis
You only have on heart, even if you have multiple kidneys and fat
β2 Adrenergic Receptors
Bronchial Smooth Muscle • Bronchodilation (reduces airway resistance)
• Some Blood Vessels • Vasodilation • Muscle, coronary arteries
• Skeletal Muscle • Stimulate glycogenolysis and lipolysis
• Liver • Stimulate glycogenolysis and gluconeogenesis and lipolysis
• Uterus • Uterine Relaxation
• GI Tract Smooth Muscle • Reduced motility
• Adipose Tissue • Stimulates Lipolysis • Pancreas • Stimulate Glucagon Secretion
Beta 2 is for scary responses, alpha receptors and Beta1 receptors are for slight changes but crank up with large stress response.significant
Regulated by hormones rather than neurons, slower
Epi-pen: helps with vasodilation when there is constriction due to an asthma attack
epinephrine primarily affects the heart rate and airway dilation, while norepinephrine primarily functions to constrict blood vessels, thereby increasing blood pressure
B2: 2 be slow, 2 be breathing
β3 Adrenergic Receptors
Adipose Tissue
• Predominantly Brown Adipose Tissue(Heat generator)
• Some in White Adipose Tissue(main adipose storage tissue)• Stimulates lipolysis • Activates thermogenesis (reduced efficiency of mitochondria)
3 = fatty
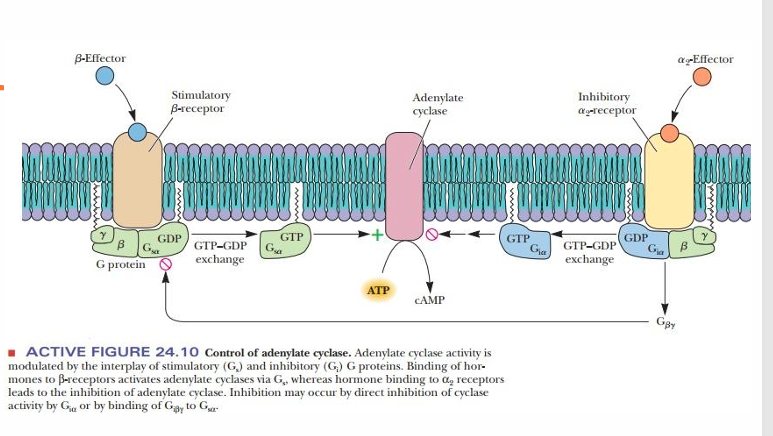
Gs and Gi GPCRs
β Receptors are Gs
α2 Receptors are Gi
• Result in activation or inhibition of Adenylate Cyclase (AC)(GS activate, GI inhibit)
AC(Adenylyl cyclase) generates cAMP • cAMP stimulates PKA (binding to regulatory proteins) • PKA phosphorylates various enzymes in the cell to activate them
AKAP and Mitochondria
• PKA Regulatory proteins can bind to AKAP (a kinase anchoring protein)
• AKAP and Phosphodiesterase (PDE) tether PKA to the mitochondrial membrane.
• cAMP then binds to PKA at the mitochondrion and is removed by PDE
• This enhances PKA function at the mitochondrion
• Phosphorylates StAR • Increases metabolite transport across the mitochondrial membrane
• Phosphorylates Cytochrome Oxidase • Increases mitochondrial efficiency and enhances Cytochrome Oxidase Activity
• β adrenergic receptor activation acutely improves mitochondrial function
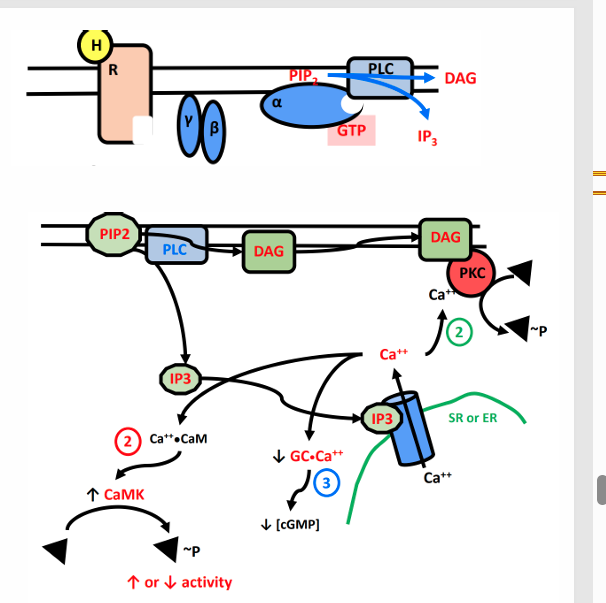
Gq GPCRs
α1 receptors
• Receptor activation leads to activation of Phospholipase C
Converts PIP2 to IP3 and DAG
• DAG activates PKC and IP3 induces calcium release from the SR
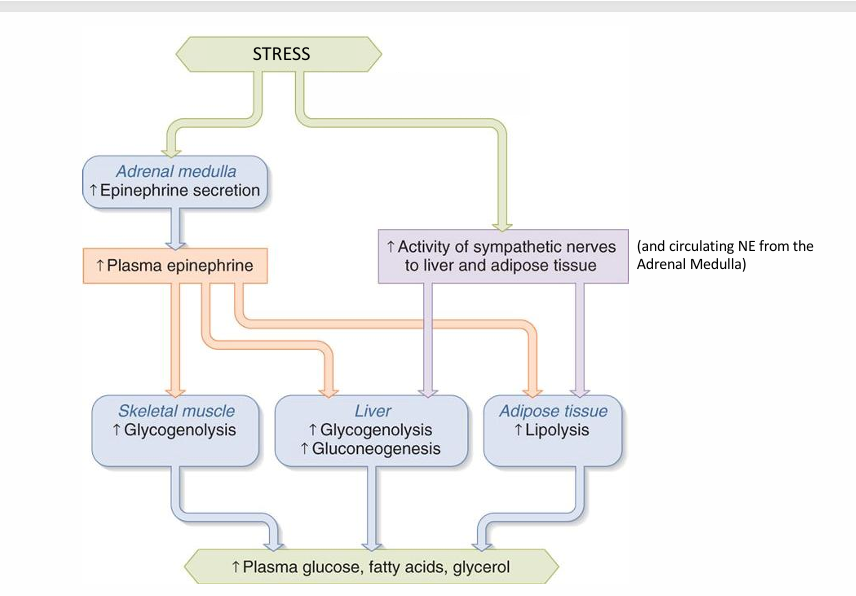
Function 1 of catecholamine: Maintain Blood Glucose
Liver increases glycogenolysis and gluconeogenesis
• Liver decreases glycogen synthesis
• Increases hepatic glucose production helps to keep blood glucose from dropping.
• SM(skeletal muscle) increases glycogenolysis (alternative to blood glucose)
Function 2: Increase availability of other fuel sources
• SM glycogenolysis • (use glycogen instead of glucose)
• SM increases lipolysis • (use fatty acids instead of glucose)
• AT increases lipolysis • (use fatty acids instead of glucose) • Increase blood fatty acid concentration for use by other tissues (including liver and SM)
• Liver Increases lipolysis • Increases fatty acid oxidation in the liver for ATP, instead of glucose
• Liver increases ketogenesis • Increased blood fatty acid concentration (from AT lipolysis) stimulates ketogenesis in the liver • Increased fatty acid oxidation in the liver stimulates ketogenesis in the liver
Function 3: Alter secretion of other hormones to aid with functions 1 & 2.
• Increased glucagon from pancreas • Increase hepatic glucose production (via glycogenolysis and gluconeogenesis)
• Decreased insulin secretion • Decrease blood glucose utilization by non-brain organs.
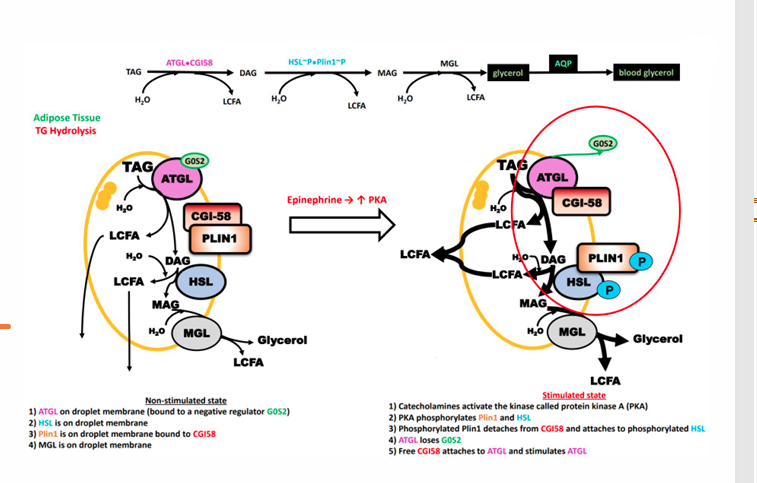
Stimulation of Lipolysis in Adipose Pathway
Epinephrine increase PKA. PKA phosphorylates PLIN1 to dissociate from CGI-58 and attach to HSL, replacing GOS2 stopping inhibition and activating ATGL and HSL to cause lipolysis in adipose tissue
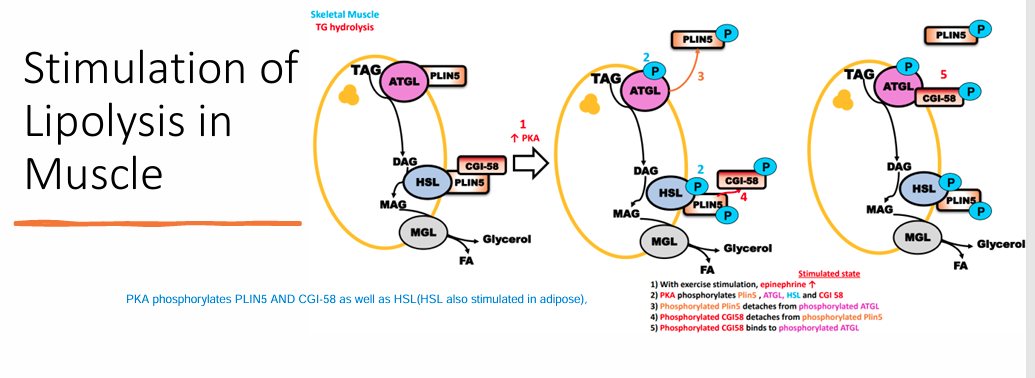
Stimulation of Lipolysis in Muscle(enzymes used)
PKA phosphorylates PLIN5 AND CGI-58 as well as HSL in order to cause lipolysis in this tissue type(HSL also stimulated in adipose),