Combustion of alkanes
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What amount of oxygen is needed for complete combustion to take place?
Excess
What amount of oxygen is needed for incomplete combustion to take place?
Insufficient
How much/ what amount of energy is released with complete combustion?
Max per mole
How much/ what amount of energy is released with incomplete combustion?
Less than max
What is the word and chemical equation for complete combustion?
fuel + oxygen → carbon dioxide + water
CH + O₂ → CO₂ + H₂O
What is the word and chemical equation for incomplete combustion?
Fuel + Oxygen → Carbon Monoxide + Carbon + Water
CH + O₂ → CO + C + H₂O
What are the health concerns with complete combustion?
None
What are the health concerns with incomplete combustion?
CO is poisonous - binds to haemoglobin
Asthma
Carcinogenic
How does Cobalt Chloride Paper demonstrate the products of combustion?
Goes from pink to blue in the presence of water
How does Lime Water demonstrate the products of combustion?
Goes colourless to cloudy in the presence of carbon dioxide
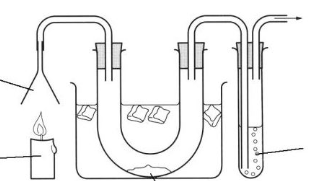
For the paractical/ demo below; 1) Explain why the funnel is upside down. 2) Explain the purpose of the water pump. 3) Explain why is the U-tube surrounded by ice.

To trap the products.
To create a pressure difference. More pressure in the test tube, less pressure in the apparatus. The pressure in the test tube pulls the gases towards the apparatus.
To condense the water.
Name the two substances used to test for water in the U-tube and describe the change in each substance that shows water is present
Cobalt Chloride: Goes from pink to blue in the presence of water
Copper Sulphate: Goes from white to blue in the presence of water
How can alkanes be distinguished from alkenes during combustion?
Alkanes burn with a clean, blue flame, while alkenes burn with a yellow, sooty flame due to incomplete combustion