2 - Nuclear Chemistry
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
What is the change in the atomic number when electron capture occurs?
decreases by 1
What is one way that nuclear reactions differ from chemical reactions?
Nuclear reactions are not affected by temperature.
What is the final product of a sequence of spontaneous nuclear decay reactions?
a stable isotope
The charge on a gamma ray is ____.
0
What is the same as a beta particle?
electron
What is the change in atomic mass when an atom emits a beta particle?
mass does not change
What is the change in atomic mass when an atom emits gamma radiation?
mass does not change
The least penetrating form of radiation is ____.
alpha radiation
Ionizing radiation identical to helium nuclei is ____.
alpha radiation
What is the change in the atomic number when an atom emits an alpha particle?
decreases by 2
What is the change in atomic number when an atom emits a beta particle?
increases by 1
What type of radiation is likely to occur when the ratio of neutrons to protons is below the band of stability?
positron emission
How does a positron differ from an electron?
A positron has a charge opposite that of an electron.
What particle decomposes to produce the electron of beta radiation?
neutron
A nitrogen isotope decays by positron emission. What element is the product of the decay?
carbon
What product of fission is necessary in order to establish a chain reaction?
two or more neutrons
A neutron breaks down to form ____.
a proton and an electron
What is a reason why spent fuel rods are stored in a pool of water?
Water acts as a radiation shield to reduce the radiation levels.
How long do spent fuel rods remain dangerously radioactive?
tens of thousands of years
What occurs when an isotope decays by the process of beta emission?
the atomic number changes
How does an atom with too many neutrons relative to protons undergo radioactive decay?
by emitting a beta particle
What particle does argon-39 (atomic number 18) emit when it decays to potassium-39 (atomic number 19)?
electron
What is the approximate half-life of uranium-238?
billions of years
A transmutation reaction must always involve a(n) ____.
change in the number of protons in a nucleus of an atom
How does the radiation from radioisotopes cause damage to human tissue?
by ionization knocking electrons away from atoms
Which particles maintain a nuclear chain reaction?
neutrons
Where do controlled nuclear chain reactions occur?
nuclear reactors
What material is commonly used to remove heat from a nuclear reactor core?
liquid sodium
A reaction in which small nuclei combine to form a heavier nucleus is a ____.
fusion reaction
nuclear fusion takes place in the ___.
sun
A reaction that results in the combining of smaller atomic nuclei is ____
fusion
What does neutron absorption accomplish in a nuclear reactor?
it slows down the reaction
Which element is used in control rods to absorb neutrons in a nuclear power plant?
cadmium
What substances are used as neutron moderators in a nuclear reactor?
carbon and water
water can be used to ____ neutrons in a nuclear reactor.
slow down
How are radioactive isotopes used to diagnose thyroid problems?
Uptake of radioactive iodine by the thyroid gland is measured.
What instrument is used routinely to check a person's exposure to radiation over a period of time?
film badge
What is the main detector of a Geiger counter?
ionizable gas in a metal tube
What is the main detector of a scintillation counter?
phosphor-covered surface
How are radioisotopes used in radiation therapy for cancer?
to kill fast growing cells
Why should a radioactive tracer used to detect diseases in internal organs emit either beta particles or gamma rays?
Alpha particles cannot travel through several millimeters of body tissue.
What is the approximate ratio of neutrons to protons for stable atoms below atomic number 20?
1:1
The half-life of radon-222 is about four days. After how many days is the amount of radon-222 equal to one-sixteenth of its original amount?
16
Above which atomic number are all nuclei radioactive?
82
What is a beta particle
an energetic electron from decomposed neutron
What is a positron?
a particle of charge +1 and mass equal to that of an electron
Damage to living cells from ionizing radiation generally occurs through disruption of
the cell’s DNA strand
The isotope ____ is frequently used in PET scanning as a radioactive label attached to glucose, which is taken up by highly active cells.
fluorine-18
The element most commonly used as fuel for nuclear fission reactors both historically and presently is
uranium
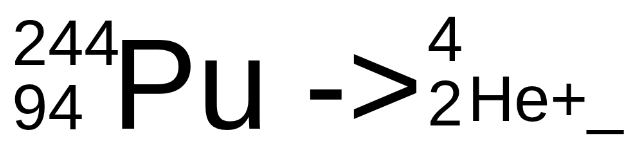
Complete the following nuclear reaction
Answer format: mass number, atomic number Element name
240, 92 U
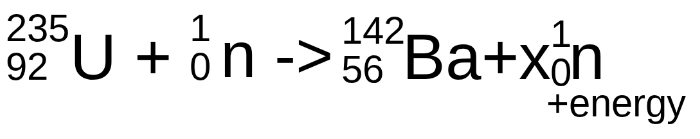
Complete the following nuclear reaction with the correct number for x
2
If the half life of a radioactive material is 8 years, how many years will it take for three fourths of the original amount of material to decay?
6 years
The concentration of carbon-14 in a piece of wood from an ancient burial mound indicates that 2.5 half lives of this radioisotope have passed. If the half life (t 1/2) for carbon-14 is 5730 years, approximately how many years ago did this sample of wood die?
14325 years
Write the nuclear equation for beta-minus decay of 210, 84 Po (format: mass number, atomic number Element name)
210, 83 Bi →210, 84 Po + 0, -1 e
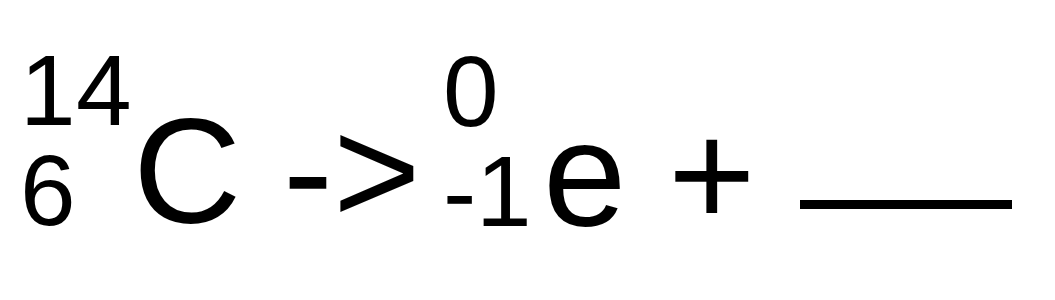
Complete the following nuclear reaction (format: mass number, atomic number Element name)
14, 5 B
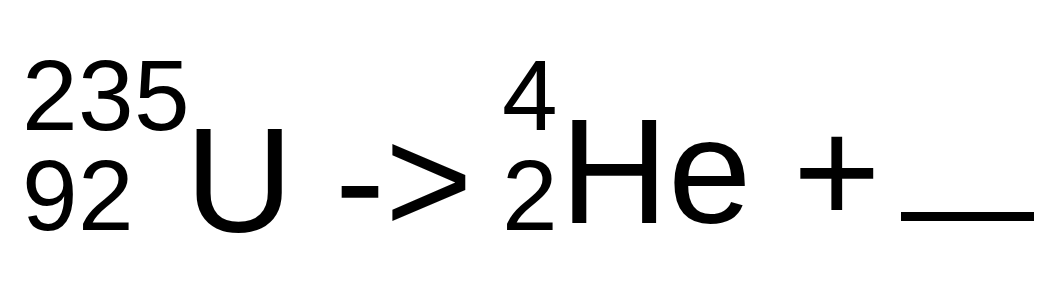
Complete the following nuclear reaction (format: mass number, atomic number Element name)
231, 90 Th
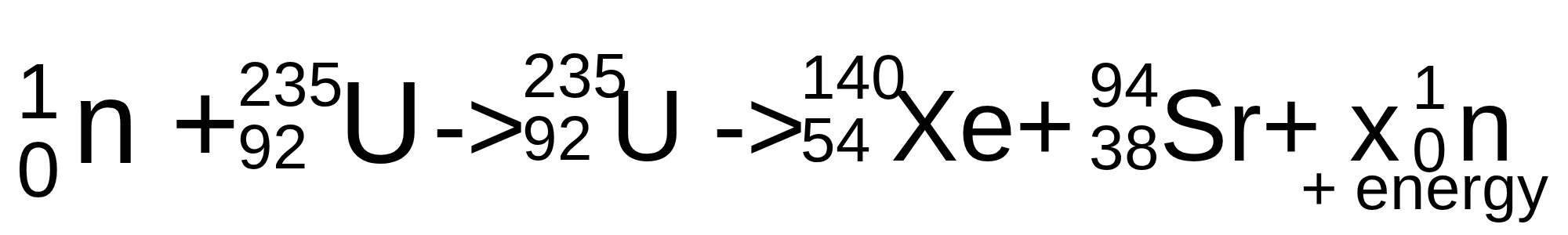
Complete the following nuclear reaction with the correct number for x
2
A medical institution requests 2g of bismuth-214, which has a half life of 20 min. How many grams of bismuth-214 must be prepared if the shipping time is 1 hour?
16g
The half life of radon-222 is about four days. After how many days is the amount of radon-222 equal to 1/16th of its original amount?
16
Iodine-131 has a half life of 8 days. If the amount of iodine-131 in a sample is 32 g, how much iodine-131 will remain after 32 days?
2g