Chapter 26: Carbonyls
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
71 Terms
what is a carbonyl functional group?
C=O
Aldehyde written in structural formula:
CHO
Ketone written in structural formula:
CO
ketone suffix:
-one
aldehyde:
-al
oxidation of aldehydes:
reflux with acidified dichromate ions
do ketones undergo oxidation reactions?
no
the C=O double bond is made up of…
a ⫪-bond
a σ-bond
is a C=C bond polar? how about a C=O double bond?
C=C = non-polar
C=O = polar → Oxygen is more electronegative than carbon
electron density in the double bond lies closer to the oxygen than to the carbon
this makes the carbon end of the bond slightly positive and the oxygen end slightly negative
In the nucleophilic attack of a carbonyl, what is the nucleophile attracted to?
the slightly positive carbon atom in the C=O double bond
What is NaBH4 used as?
a reducing agent?
NaBH4 + aldehyde →
reduced to a primary alcohol
reduction of an aldehyde equation:
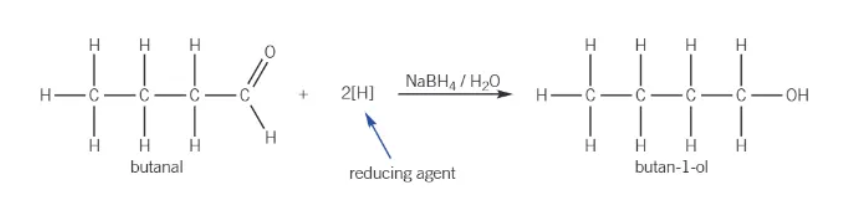
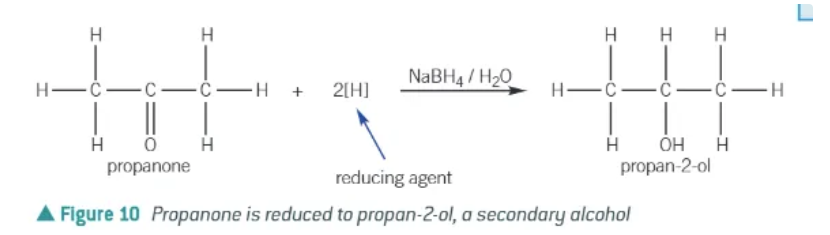
what are ketones reduced by NaBH4?
secondary alcohols
how are reducing agents represented?
[H]
Hydrogen Cyanide:
colourless
extremely poisonous
boils slightly above room temperature
carbonyl + HCN →
hydroxynitrile (requires H2SO4 / HCN)
reaction conditions for the reduction of carbonyls:
NaBH4 / H2O
reaction conditions for the reaction of HCN + carbonyl
H2SO4 / NaCN
NaBH4 contains what ion, acting as a nucleophile?
hydride ion → :H-
mechanism for the reaction of NaBH4 with a carbonyl:
lone pair from hydride ion, :H-, is attracted and donated to the δ+ carbon atom in the aldehyde or ketone C=O double bond.
a dative covalent bond is formed between the hydride ion and the carbon atom of the C=O double bond.
The ⫪-bond of the C=O double bond breaks by heterolytic fission, forming a negatively charged intermediate.
The oxygen atom of the intermediate donates a lone pair of electrons to a hydrogen atom in water molecule.
The intermediate has been then protonated to form an alcohol.
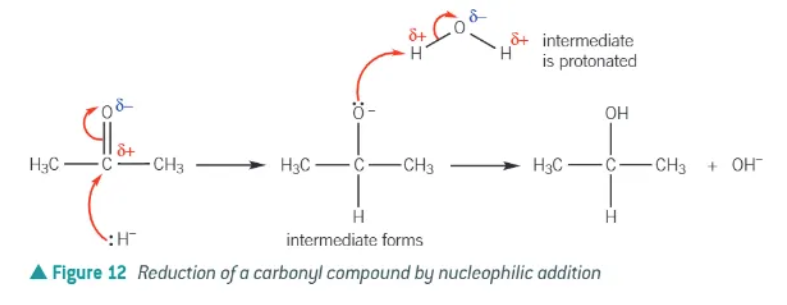
drawn out mechanism for the reduction of a carbonyl with NaBH4:
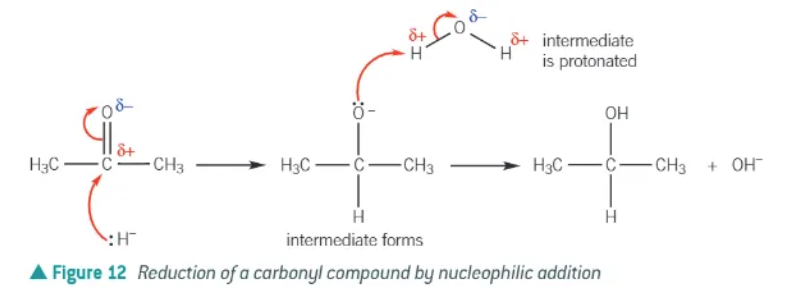
addition of a carbonyl compound usually consists of 2 steps:
nucleophilic attack on the carbonyl group to form a negatively charged intermediate
protonation of the intermediate to form an alcohol
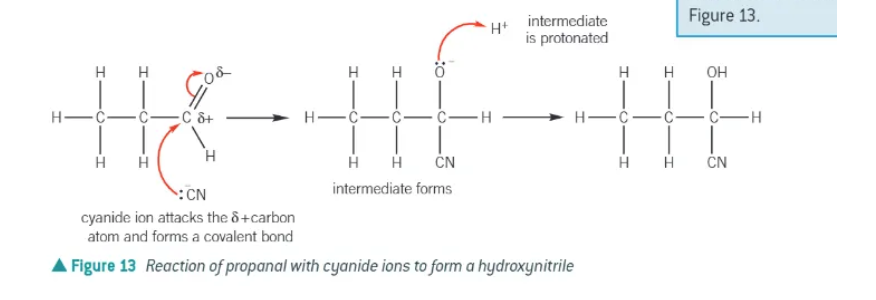
reaction of NaCN/H+ steps:
long pair of electrons from the cyanide ion, :CN-, is attracted and donated to the δ+ carbon atom in the aldehyde or ketone C=O double bond. A dative covalent bond forms.
The ⫪-bond in the C=O double bond breaks by heterolytic fission, forming a negatively charged intermediate.
The intermediate is protonated by donating a lone pair of electrons to a hydrogen ion, to form the product.
The product is a hydroxynitrile.
which atom is the negative charge of a cyanide ion on?
the carbon → -CN → needs to be shown in the mechanism
drawn out mechanism of HCN + carbonyl:
what is used to detect a carbonyl group? what colour does it go?
2,4-dinitrophenlyhydrazine → 2,4-DNP
forms a yellow or orange precipitate
In practical work, 2,4-DNP is usually dissolved in…
in methanol and sulphuric acid as a pale orange solution called Brady’s reagent.
Once 2,4-DNP has been used to establish that a compound is a carbonyl, how can we distinguish between aldehydes and ketones?
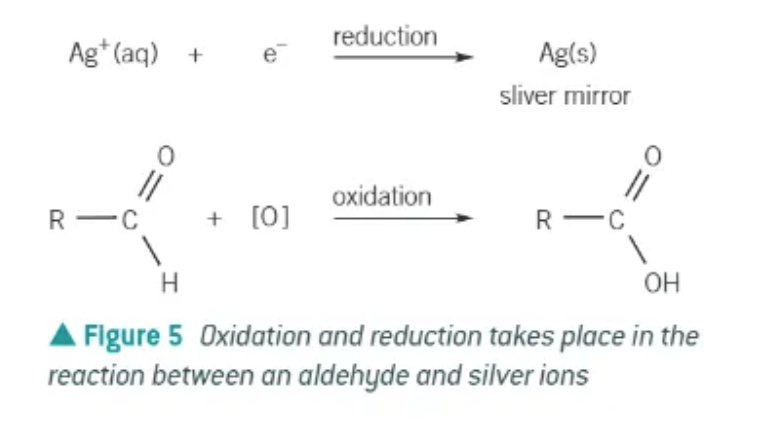
Tollen’s reagent → solution of silver nitrate in aqueous ammonia.
aldehyde = silver mirror is produced
2 reactions take place during the use of a Tollens’ reagent:
oxidation of aldehyde → carboxylic acid
reduction of Ag+(aq) ions in the Tollens’ reagent to form Ag(s) (silver mirror)
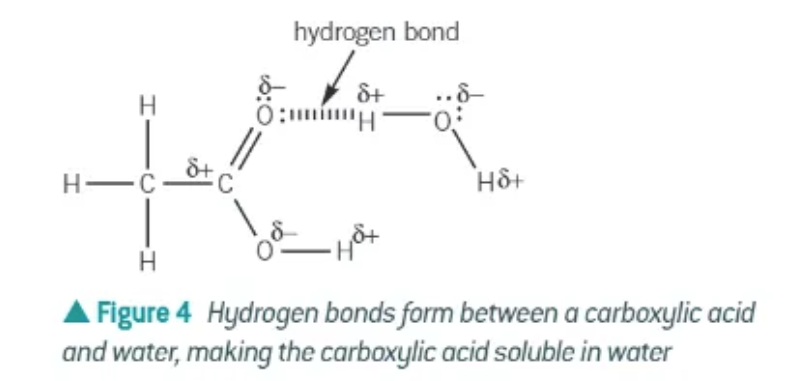
solubility of carboxylic acids:
the C=O and O-H bonds in carboxylic acids are polar, allowing carboxylic acids to form hydrogen bonds with water molecules.
Carboxylic acids with up to 4 carbon atoms are soluble in water.
As the number of carbon atoms increases, the solubility decreases as the non-polar chain has a greater effect on the overall polarity of the molecule.
what are even more soluble carboxylic acids?
dicarboxylic acids
carboxylic acids are classified as … acids
weak
when dissolved in water, methanoic acid partially dissolves in water. Draw the reversible reaction established:
HCOOH(aq) ⇌ H+(aq) + HCOO-(aq)
carboxylic acids take place in redox and neutralisation reactions. What are these, and with what?
redox with metals
neutralisation with bases (alkalis, metal oxides, carbonates)
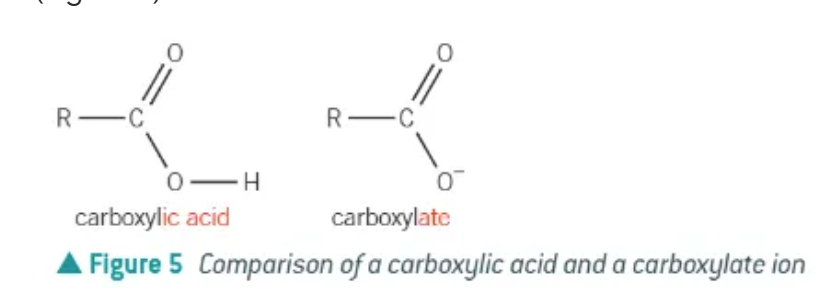
After being involved in a neutralisation / redox reaction, what does a carboxylic acid form?
carboxylate salts → COO-
neutralisation reaction of carboxylic acids with bases:
carboxylic acids react with all bases → metals, alkalis, carbonates.
redox reactions of carboxylic acids with metals:
aqueous solutions of carboxylic acids react with metals in a redox reaction to form hydrogen gas and the carboxylate salt.
metal = disappears
hydrogen gas = produced
2CH3CH3COOH(aq) + Mg(s) → (CH3CH2COO-)2Mg2+(aq) + H2(g)
carboxylic acids + metal oxides →
salt + water
2CH3COO(aq) + CaO(s) → (CH3COO-)2Ca2+(aq) + H2O(l)
carboxylic acid + alkalis →
salt + water
overall: CH3COOH(aq) + NaOH(aq) → CH3COO-Na+(aq) + H2O(l)
ionic: H+(aq) + OH-(aq) → H2O(l)
carboxylic acid + carbonate →
salt + water + carbon dioxide
2CH3COOH(aq) + Na2CO3(aq) → 2CH3COO-Na+(aq) + H2O(l) + CO2(g)
text for the carboxyl group?
compound + carbonate → carbon dioxide
what is a derivative of a carboxylic acid?
a compound that can be hydrolysed to from the parent carboxylic acid.
carboxylic acids have a common sequence of atoms in their structure:
acyl group
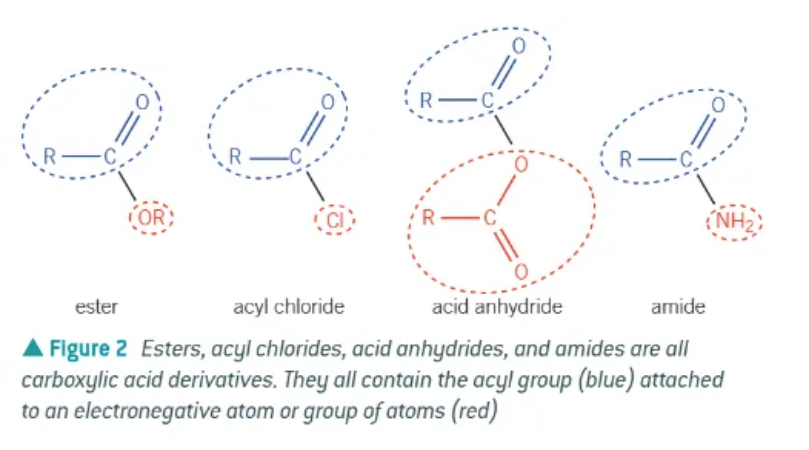
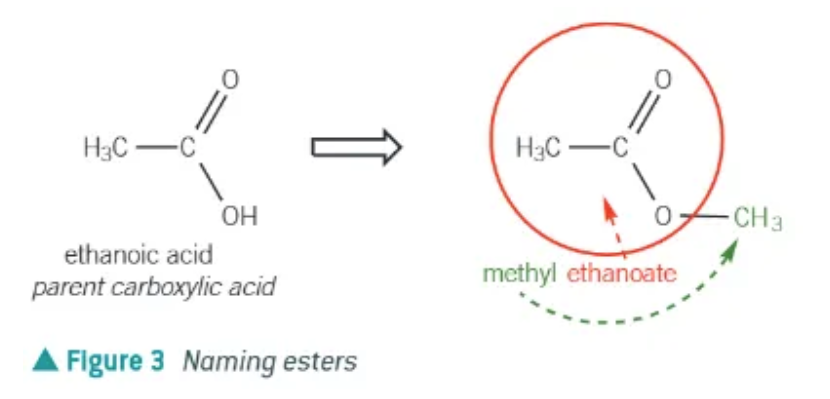
in an ester, the name comes from
prefix = alcohol
suffix = carboxylic acid
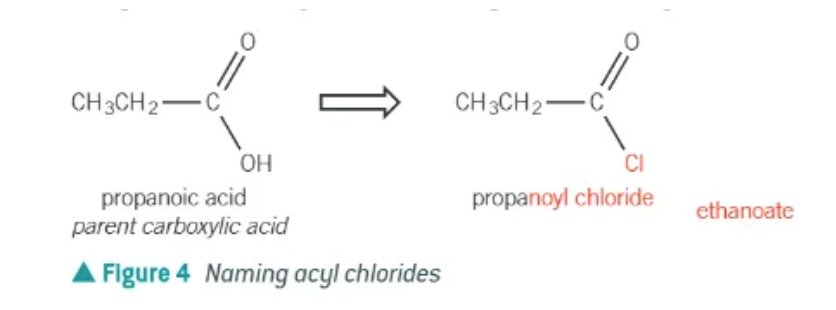
naming of an acyl chloride:
remove the -oic acid suffix from the parent carboxylic acid and replace with -oyl chloride
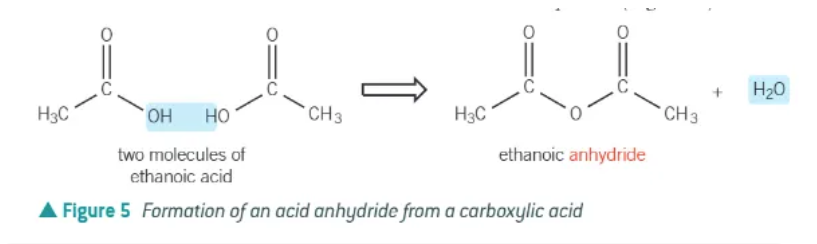
acid anhydride:
removal of water molecules from 2 carboxylic acid molecules
an -OH taken from one
an H+ from the other
esterification:
alcohol + carboxylic acid → ester + water (with conc. sulphuric acid catalyst)
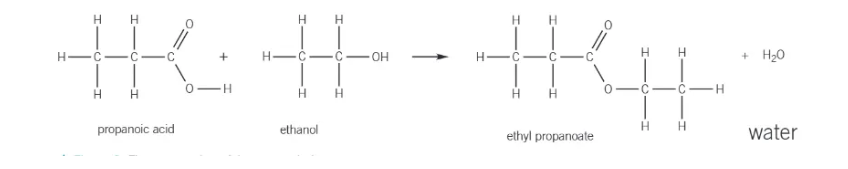
reaction conditions for esterification
small amount of concentrated sulphuric acid → catalyst
what hydrolyses esters?
aqueous acid or alkali
what is hydrolysis?
the chemical breakdown of a compound in the presence of water or in an aqueous solution
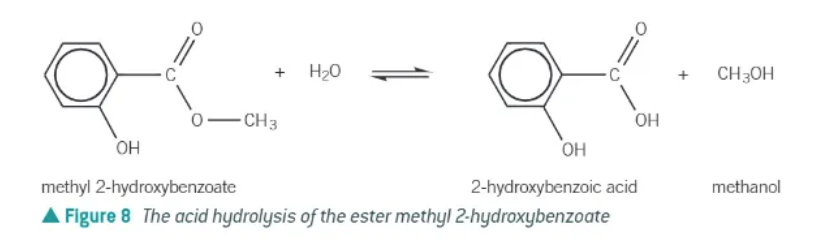
acid hydrolysis:
reverse of esterification
heat ester under reflux with dilute aqueous acid
ester broken down by water, with acid acting as catalyst
products = carboxylic acid + alcohol
reversible
alkaline hydrolysis:
aka saponification and is irreversible
ester is heated under reflux with aqueous hydroxide ions
forms carboxylate ion and an alcohol
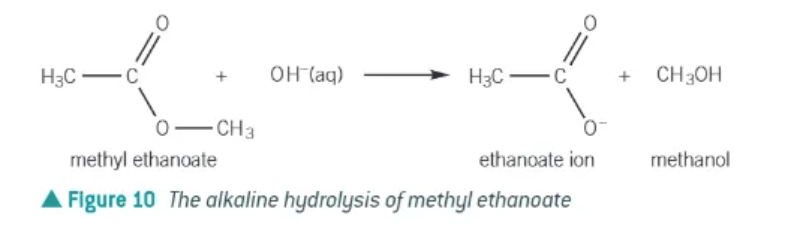
alkaline hydrolysis: NaOH + methyl ethanoate →
CH3COO-Na+ + CH3OH
preparation of acyl chloride (should be done in a fume cupboard)
parent carboxylic acid + thionyl chloride (SOCl2) → acyl chloride + SO2(g) + HCl(g)
acyl chloride + alcohol →
ester + HCl
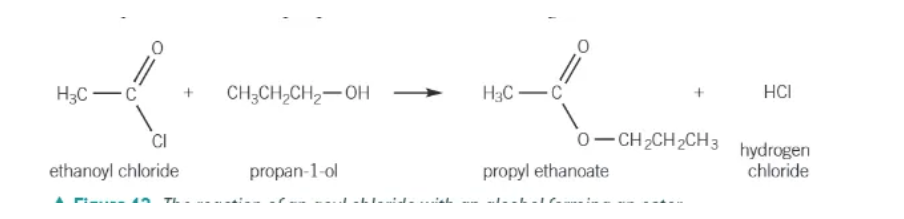
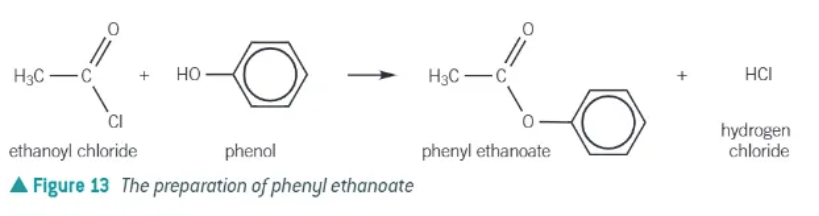
acyl chloride + phenols →
phenyl ester + HCl
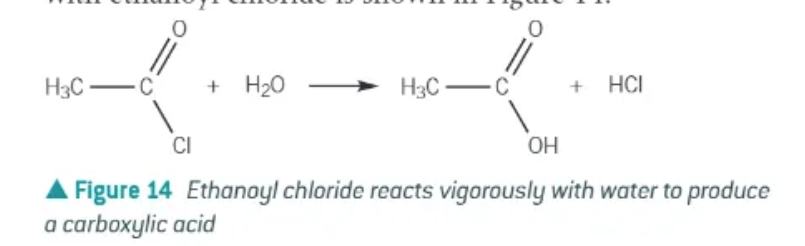
acyl chloride + water →
carboxylic acid + HCl gas
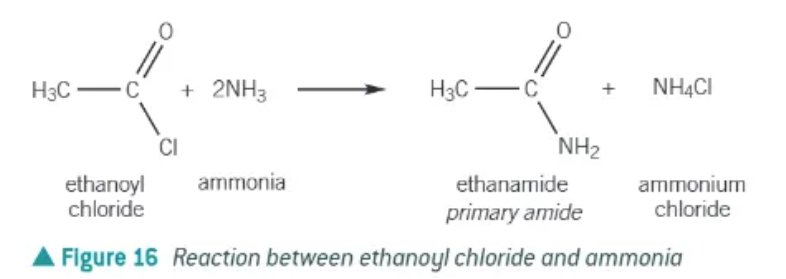
acyl chlorides + ammonia →
primary amide + ammonium chloride
(the ammonia is a nucleophile)
amide group:
-NH2
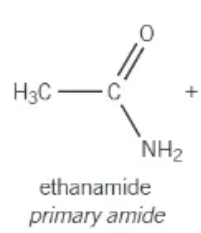
primary amide:
the nitrogen atom is attached to 1 carbon atom
what does the name N-methylamide show us?
the methyl group is attached to the nitrogen rather than a carbon atom of the main carbon chain
2 x primary amine + acyl chloride →
secondary amide + ammonium chloride salt
ethanoyl chloride + methylamine →
N-methylethanamide + methylammonium chloride
acyl chlorides are…
very reactive
acid anhydrides are…
less reactive than acyl chlorides:
acid anhydride + alcohol →
ester + carboxylic acid
mechanism of acyl chlorides + nucleophiles →
addition then elimination
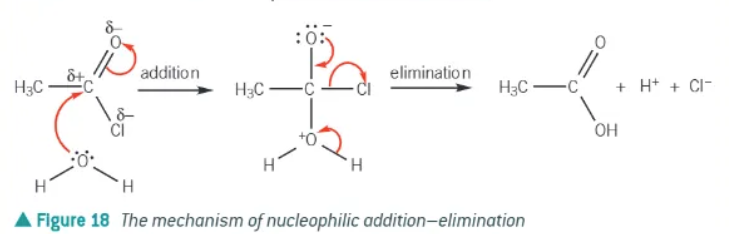
if NaOH is used as the alkali in alkaline hydrolysis, what forms?
alcohol + salt: e.g. sodium ethanoate
acid anhydrides react with …
-OH groups
acid anhydrides give…