module 2 - foundations in chemistry
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
What conditions does the ideal gas equation presume are in place to calculate with it?
- no forces of attraction between particles
- gas particles have no volume
- random motion
- elastic collisions - no loss of energy from particles
What shape is formed when there are 3 bonding pairs of electrons (+ one lone pair) ?
Pyramidal :
- 3 bonding pairs but also can contain 1 lone pair
- e.g Ammonia
- Bond angles decrease from tetrahedral to pyramidal so they = 107

Why are non-polar simple molecular substances soluble in non-polar solvents?
London forces between the molecules and the solvent are able to form causing the SMSs induced dipole-dipole interactions in the molecular lattice to weaken making it dissolve.
Why can polar molecules be soluble in polar solvents?
In general polar SMSs will dissolve in polar solvents because the polar molecules from the solvent & solute are able to form permanent dipole-dipole interactions (or hydrogen bonds) between the.
What are some oxidation numbers for atoms in special cases?
H in metal hydrides - -1
O in peroxides - -1
O bonded to F - +2
What do electrons orbit the nucleus in?
In energy levels which increase in distance around the nucleus as the amount of energy in each level increases.
What is the structure of metals in metallic bonding?
Giant metallic lattice:
Cations are fixed in position maintaining the structure of the metal.
The delocalised electrons are mobile and can move throughout the whole structure.
How would you find the empirical formula from the mass of a molecule?
- Find the moles of each element
- Find the smallest whole number ratio by dividing all of the moles of the elements by the smallest whole number
-Use the ratio to write out the empirical formula
How are covalent bonds formed?
When orbitals each containing one electron overlap forming a molecular orbital where an electron pair can be found.
- the more overlap there is the stronger the covalent bond
What is electron-pair repulsion theory?
- Each pair of electrons around an atom will repel all other electron pairs
- The pairs of electrons will therefore take up positions as far apart as possible to minimise repulsion which effects the shape of the molecule

Why does electronegativity decrease down a group?
As you move down a group, the radius increases as more electrons shells are added. Since the outer electrons (those involved in bonding) are farther from the nucleus, they will feel the "pull" of the nucleus less. Larger atoms have lower electronegativity.
What are permanent dipoles?
A dipole in a polar covalent bond which doesn't change - permanent dipole is used to separate it from induced dipoles.
How can a molecule have permanent dipoles but be a non-polar molecule?
If the shape of the molecule is symmetrical then the dipoles act in opposite directions and so exactly oppose each other and cancel out.
What is a base?
a substance that releases hydroxide ions when in an aqueous solution and accepts H+ ions from acids to form a salt
What are some bases & alkalis?
Metal oxides, metal hydroxides, metal carbonates and ammonia
Why are ionic compounds soluble?
An ionic lattice will dissolve in polar solvents e.g. water so water molecules are able to break down the lattice and attract the cations and anions
What are isotopes?
Atoms of the same element with different numbers of neutrons and so different masses.
What happens when an isotope has a higher mass number?
Isotopes with a higher mass number have slightly different physical properties :
- increasing the mass increases the boiling point and the melting point.
- increased density
There is no change in the way they react as they still have the same number of electrons.
What is a cation?
A positively charged ion - one which has lost electrons

What is an anion?
A negatively charged ion - one which has gained electrons

What is the relative mass of an electron?
1/1836
What is the relative mass of an isotope relative to?
A carbon-12 isotope. We know exactly how much it weighs so everything is relative to it.
What is an atomic mass unit?
1/12 the mass of a Carbon-12 atom. A single unit describes the relative mass of a proton or neutron as 12us = carbon-12/6 protons + 6 neutrons.
What is relative isotopic mass?
The mass of an isotope relative to 1/12th of the mass of an atom of carbon-12
* relative isotopic mass is unique to an isotope not the mass number
What is relative atomic mass?
The weighted mean mass of an atom of an element relative to 1/12th of the mass of an atom of carbon-12
What is mass spectrometry used for?
- find the abundance and mass of each isotope in an element allowing us to determine its relative atomic mass
- to find relative atomic masses and their relative abundances
How does a mass spectrometer work?
- A sample is placed into the mass spectrometer
- The sample is vaporised and then ionised to form 1+ cations
- The ions are accelerated so the ions of different masses/each isotope are separated by their masses
- The ions are detected as a mass-to-charge ratio =
relative mass of ion/relative charge
- for cations a 1+ charge makes the result the same as their relative mass
How do you find the relative atomic mass?
sum of (isotope mass x isotope abundance) / sum of abundances
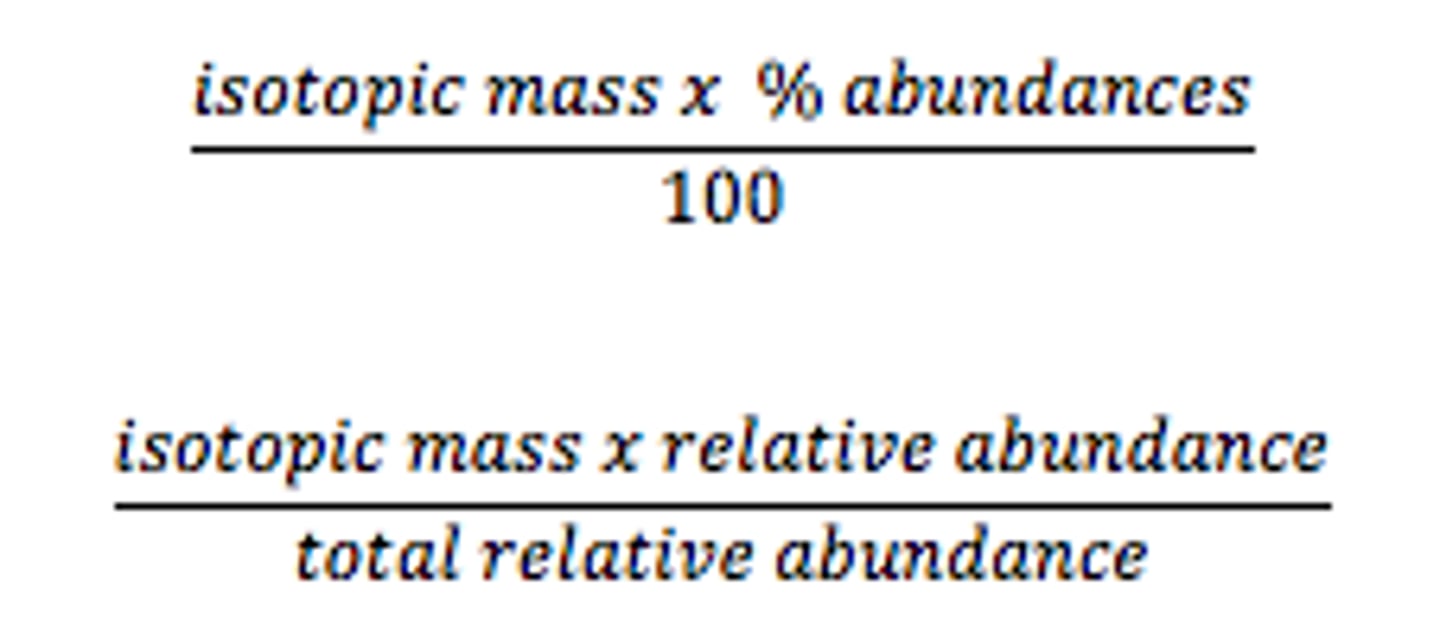
How many electrons can there be each shell (up to 4) ?
1st shell - 2 electrons
2nd shell - 8 electrons
3rd shell - 18 electrons
4th shell - 32 electrons
What is the principal quantum number?
n - the higher n is the higher the energy level
What is the difference between a shell and an atomic orbital?
A shell is a group of atomic orbitals.
An atomic orbital is a region of space around the nucleus where only 2 electrons with the opposite spin are like to be found.
What are the different types of atomic orbitals and the differences between them?
s- orbital - sphere shape, found in shells from n=1
p - orbital - dumb-bell shape, found in shells from n = 2
d - orbital - found in shells from n = 3
f - orbital - found in shells from n = 4
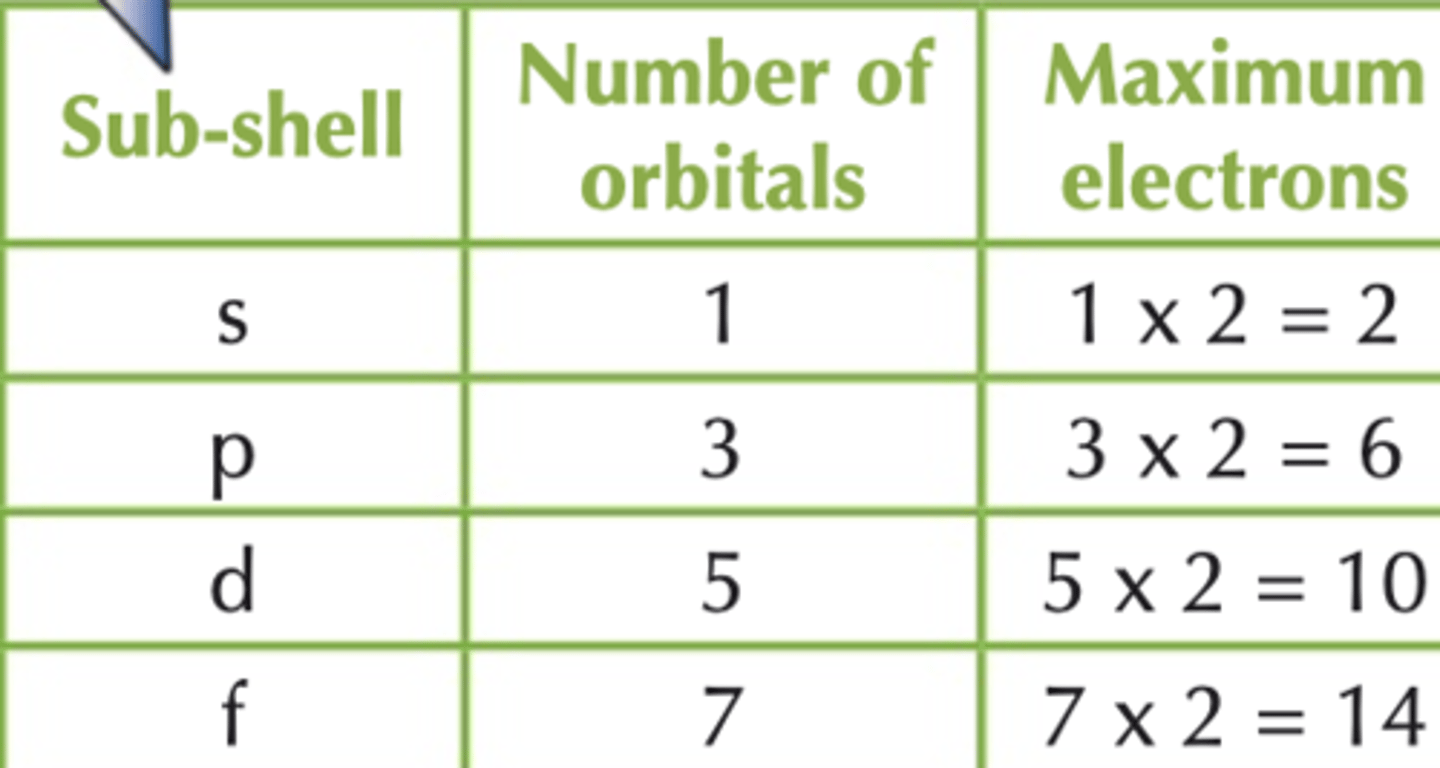
How many electrons can there be in each type of orbitals sub-shell?
s-orbital sub shell - 2 electrons
p-orbital subshell - 6 electrons
d-orbital subshell - 10 electrons
f-orbital subshell - 14 electrons
How many orbitals are there in each type of sub-shell?
s subshell = 1 orbital
p subshell = 3 orbitals
d subshell = 5 orbitals
f subshell = 7 orbitals
What is notation for electron configuration?
2s = shell number & orbital type
s² = orbital type & number of electrons
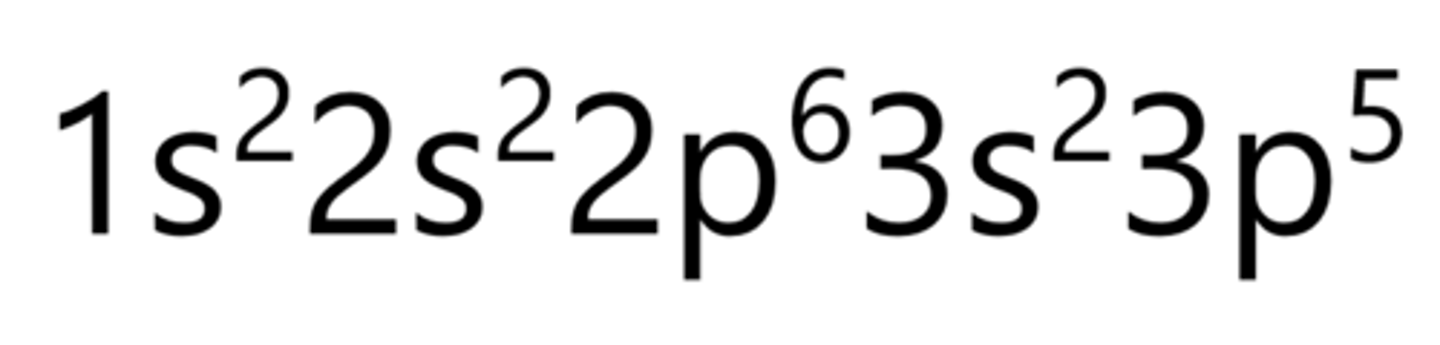
In a box diagram what do the boxes represent?
The boxes represent atomic orbitals. Each box represents 2 electrons.
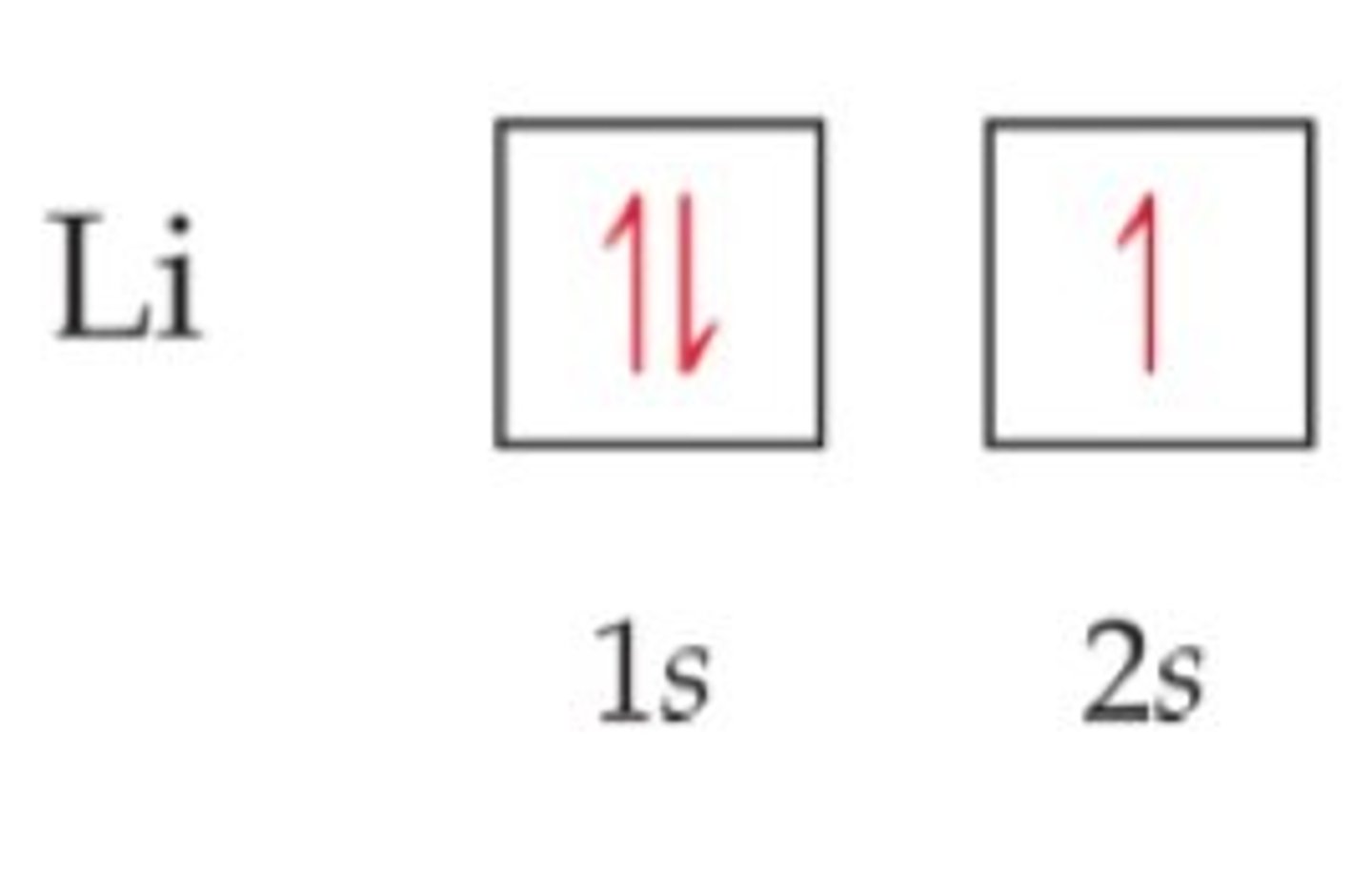
What are the different rules to box diagrams?
Pauli Exclusion Principal - each electrons can contain 2 electrons and the spins of the electrons must be opposing.
Hund's rule - single electrons will fill empty orbitals available and then start to form pairs
Aufbau principle - lower energy orbitals are filled first - 4s is a lower energy orbital so it's filled first before 3d but the 4s electrons are also lost first
What are the two exception to the rules of atomic orbitals?
Chromium & copper. Instead of two electrons pairing in the 4s shell like 4s² they have unpaired 4s block so Chromium end in 4s¹ 3d ⁵ and Copper ends in 4s¹ 3d¹⁰
What is the empirical formula?
The simplest whole number ratio of atoms for each element present in a compound
What is the molecular formula?
The number of atoms of each element in a molecule/compound as it is found naturally
What is the difference between hydride and a hydrogen ion?
hydride =H-
hydrogen cation = H+
How do you get from percentage composition to empirical formula?
Divide the percentages by the Ar to get the mole ratios
Round the ratios to the nearest integer
Use ratios to find formula e.g. 2:1 ratio = H₂0
What is the equation for the moles of a gas at room temperature and pressure?
At 298K and 100kPa, 1 mole of any gas occupies 24 dm³/24000 cm³
= moles = volume of gas/ 24 or 24000
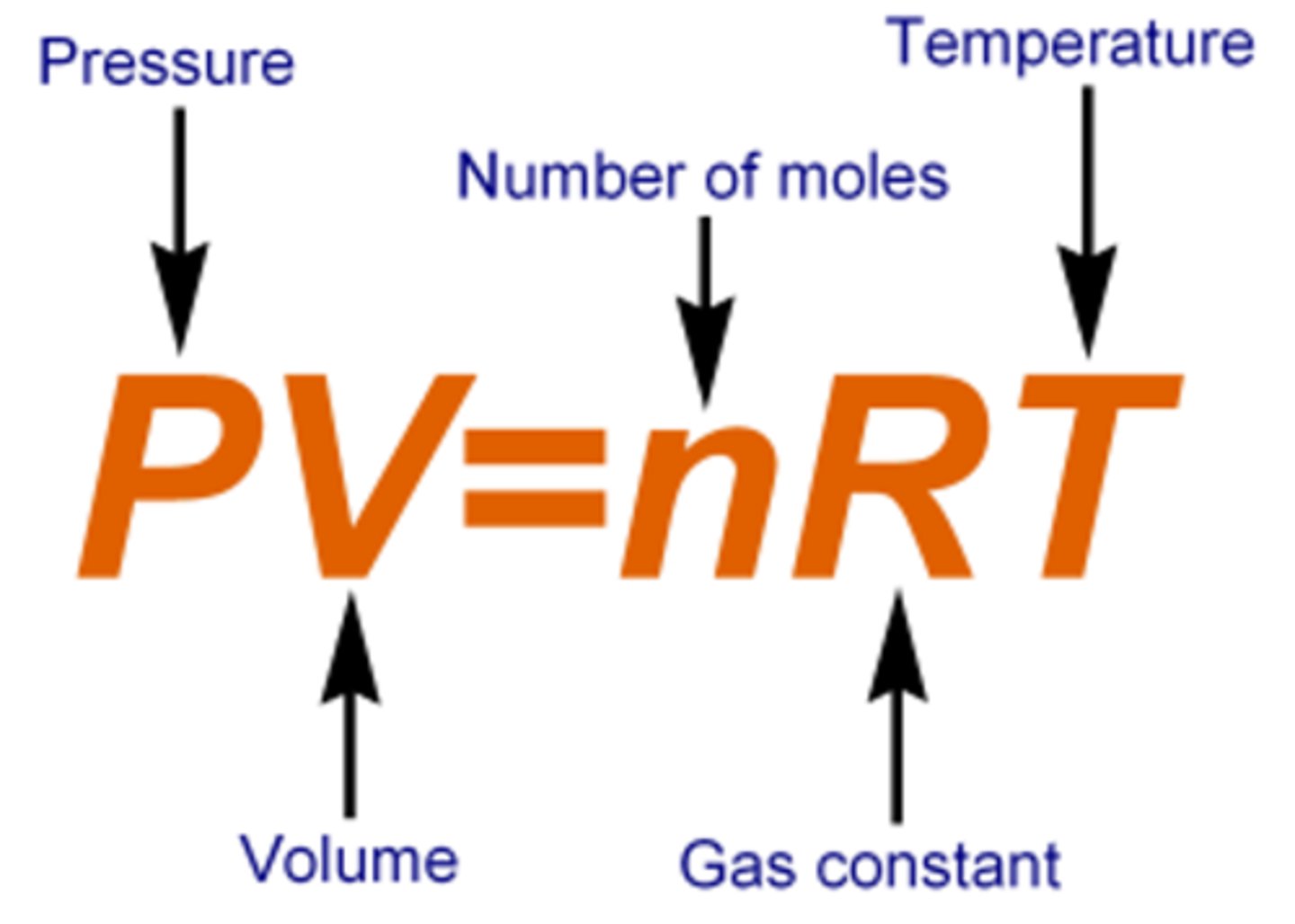
What is the ideal gas equation?
p = pressure in pascals
V = volume in m³
n = number of moles
R = 8.31 the gas constant
T = temperature measured in kelvin
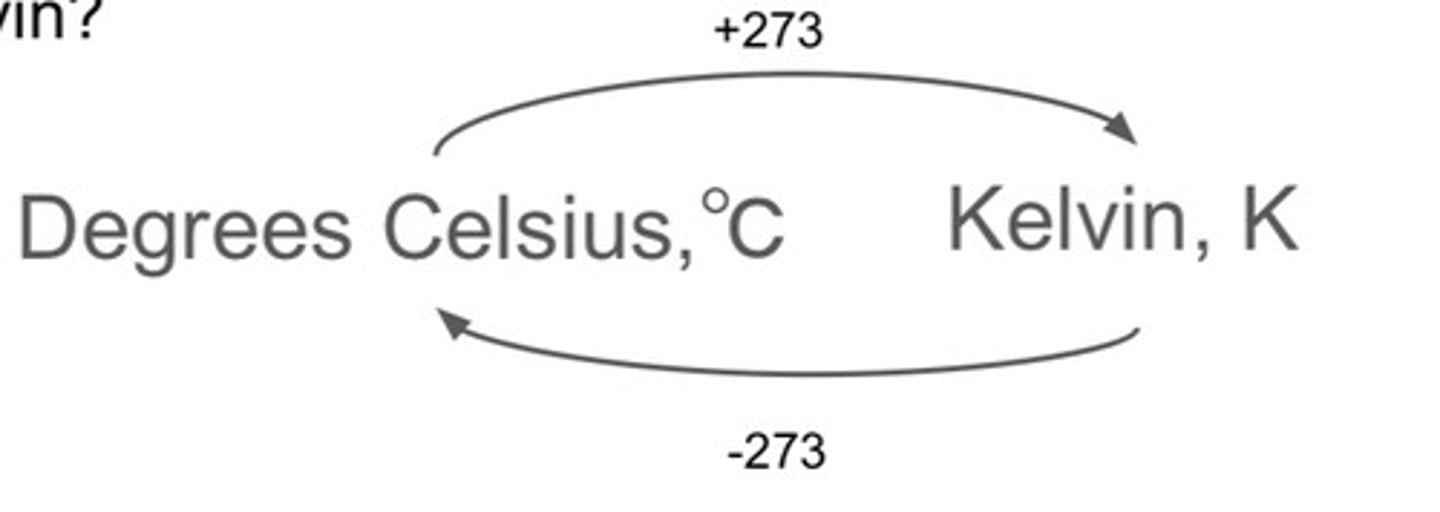
How do you convert from Kelvin to Celsius and back?
K ----> C = 273K - x degrees celsius
C ----> K = x degrees celsius + 273K
What is the equation for moles from a volume and concentration?
moles = volume x concentration
mol = mol/dm³ x dm³
or when using cm³ :
moles = vol (cm³)/1000 x volume (dm³)
What is ionic bonding?
The electrostatic attraction between positive and negative ions which acts in all directions ( to form giant ionic lattices)
Which elements don't form ions?
Boron, carbon,silicon
What are the properties of ionic compounds/ giant ionic lattices?
-solids at room temp
-high melting and boiling points
-conduct electricity when molten or dissolved in water
-they are soluble
Why are ionic compounds solids at room temp and have high melting & boiling points?
A lot of thermal energy is needed to overcome the strong electrostatic attraction between each of the ions.
What effects the strength of the ionic bond?
- the charge of the ions - as it increases so does the strength
- size of the ion - when it decreases the strength increases
Why can ionic compounds only conduct electricity when molten or dissolved?
The ions need to be mobile to carry a charge which only occurs when they’re molten or dissolved.
What is metallic bonding?
Strong electrostatic attraction between cations and delocalised outer shell electrons which have been donated by the cations to a shared pool of electrons
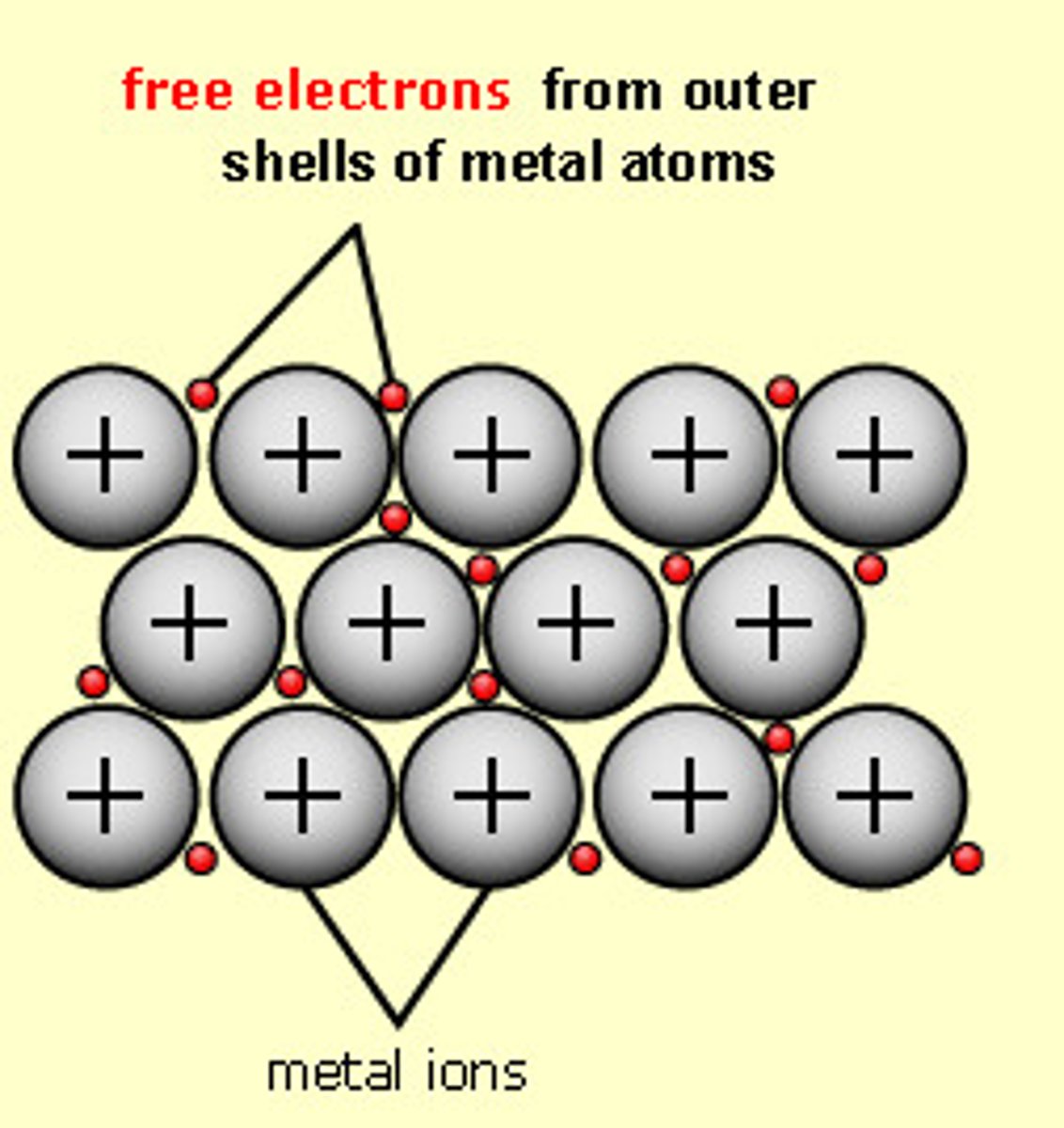
What are the properties of metals?
- electrical conductivity
- high melting and boiling points
- insolubility
How are metals able to conduct electricity?
As solids and liquids metals have mobile delocalised electrons which are able to move while carrying a charge
Why do metals have high melting and boiling points?
High temps/ lots of thermal energy is required to overcome the strong electrostatic attraction between cations and delocalised electrons - this depends on the strength of the metallic bond
What effects the strength of the metallic bond?
- number of delocalised electrons per cation - the more there are the stronger the bond
- the size of the ion - the smaller it is the stronger the bond
- the charge density - the higher it is the stronger the bond
Why are metals insoluble?
the metallic bonds are too strong and polar solvents like water can't interrupt the giant metallic lattice
What is the equation for moles?
Moles = mass/Mr

What is a covalent bond?
The localised electrostatic attraction between only a shared pair of electrons and the nuclei of the bonded atoms.
What is a lone pair of electrons?
An unshared pair of electrons /electrons that are not involved in bonding as to not exceed the octet rule
What is the octet rule?
the tendency of atoms to prefer to have eight electrons in the valence shell/outer most shell
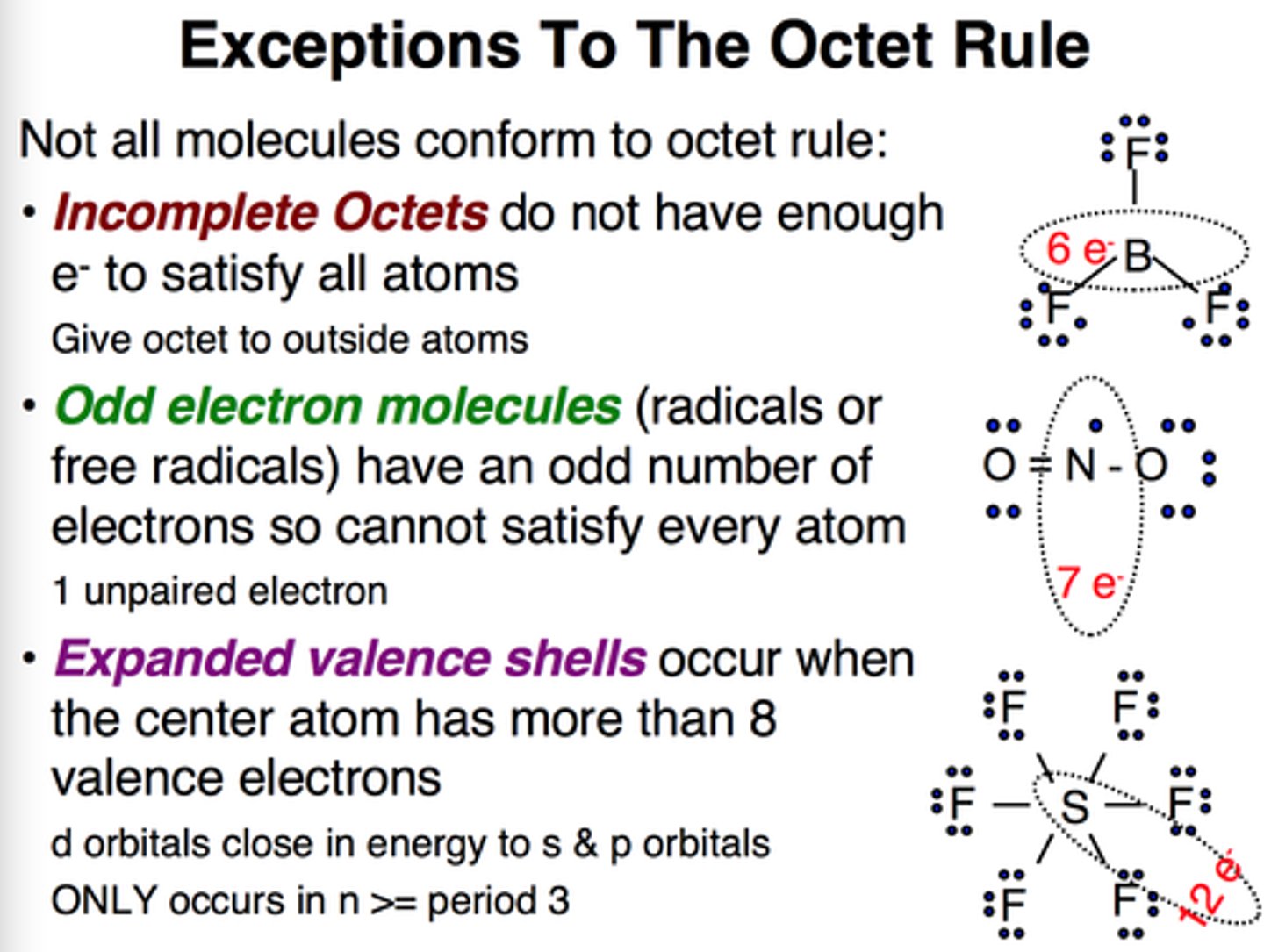
What are the main exceptions to the octet rule?
Boron - is fine forming compounds like BF3 because each electron in it's outer shell is paired/bonded - less than the octet rule
Sulfur (phosphorous& chlorine) - electron configuration (6 outer shell electrons) mean it can form up to 6 bonds as some electrons can move to the 3n shell - expands the octet rule
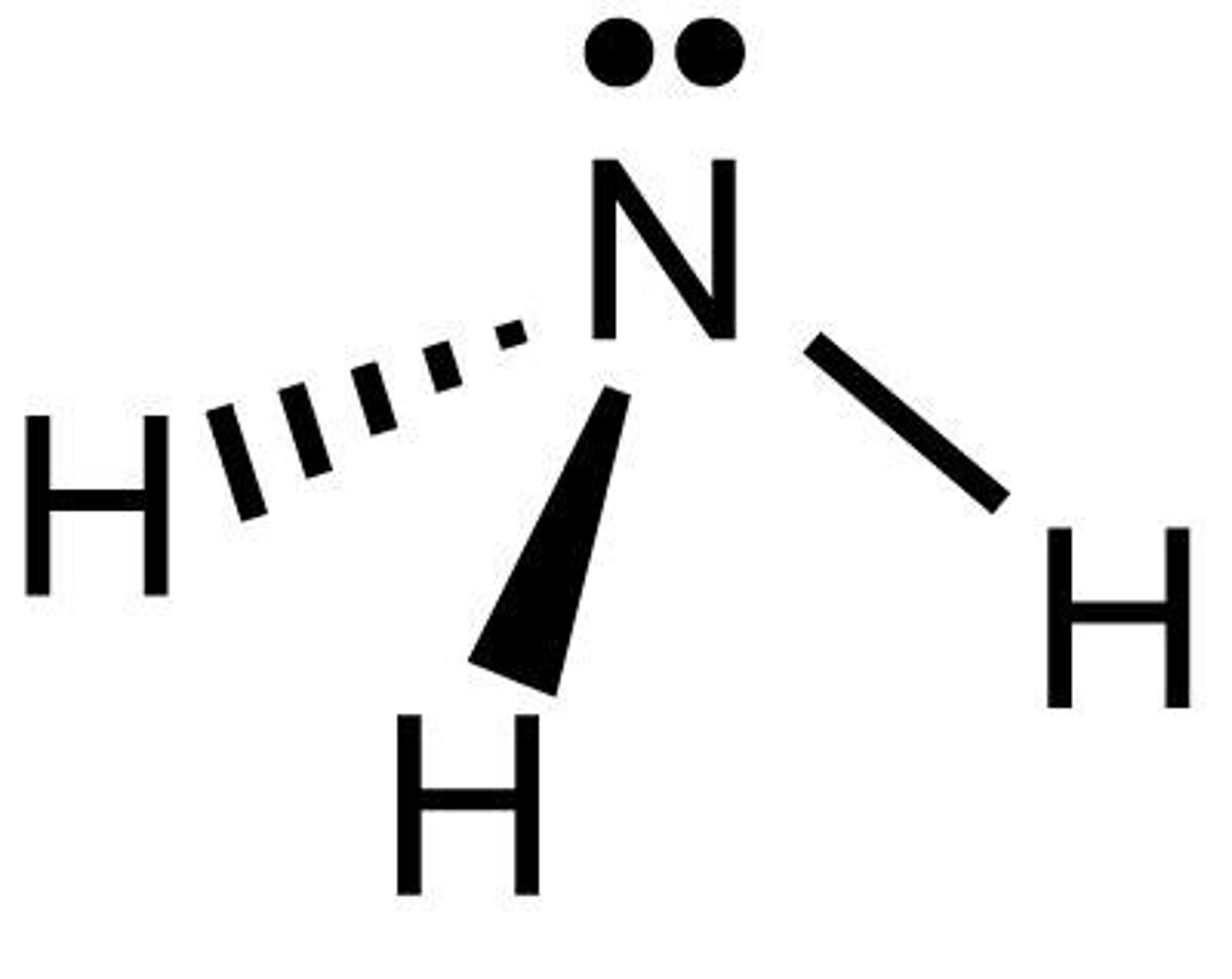
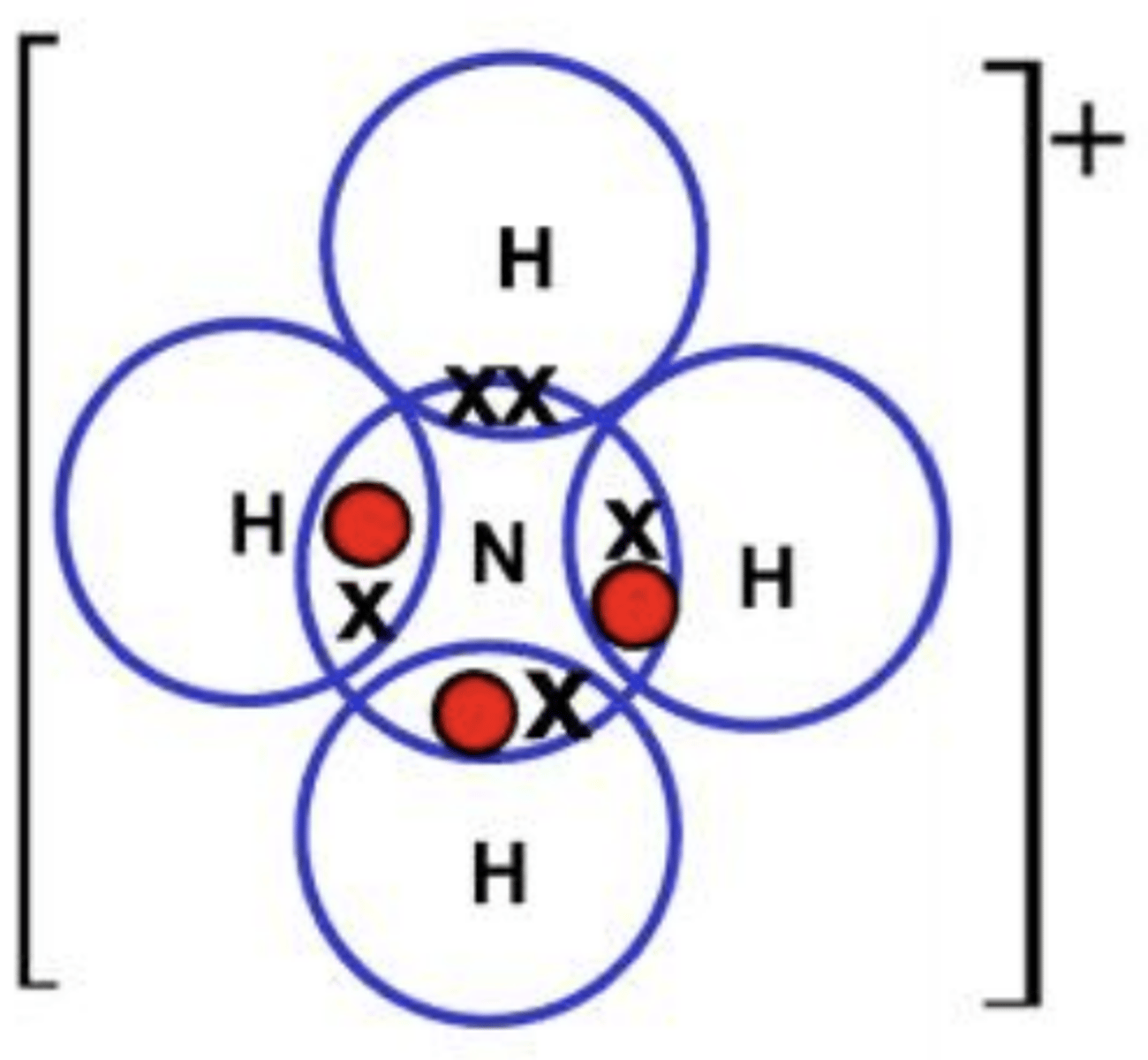
What are dative covalent bonds?
a covalent bond (a shared pair of electrons) in which both electrons come from the same atom - likely to happen when there's a lone pair
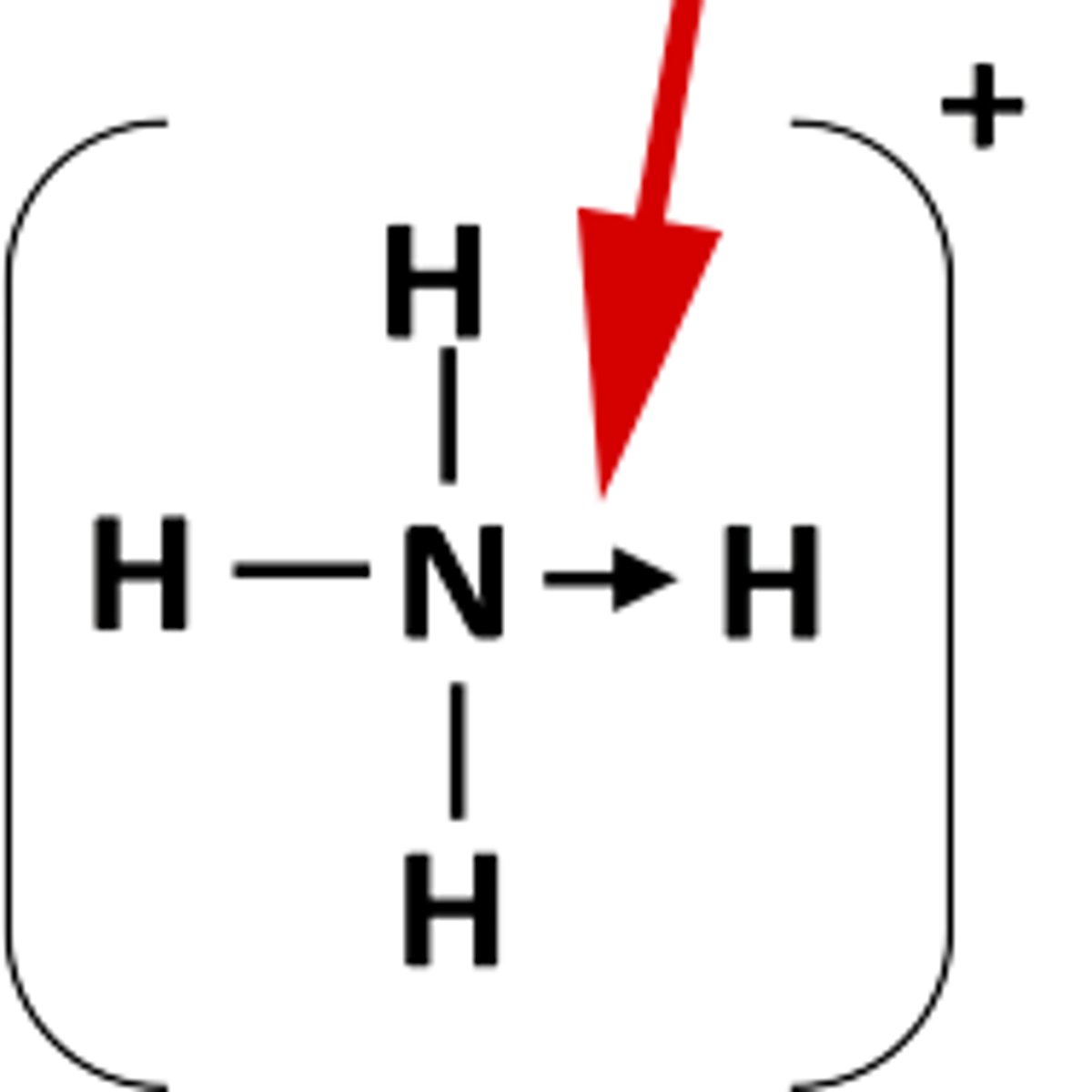
How is a dative bond represented?
arrow = N is giving electrons in a dative bond
What are simple molecular substances?
They contain only a few atoms held together by covalent bonds and have weak intermolecular forces between them forming a simple molecular lattice structure.

What are the properties of a simple molecular lattice?
- low melting and boiling point - weak intermolecular forces
- cannot conduct electricity - no charged particles which are mobile
What are the 3 elements which form giant covalent lattices?
Boron, Carbon, Silicon (as silicon dioxide)
How are giant covalent lattices and simple molecular lattices different?
Giant covalent lattices have strong covalent bonds between each atom meaning they have high melting & boiling points, (most) cannot conduct electricity and are insoluble in any solvent (polar or non-polar).
Simple covalent lattices have weak intermolecular forces between the molecules so they have low melting & boiling points.
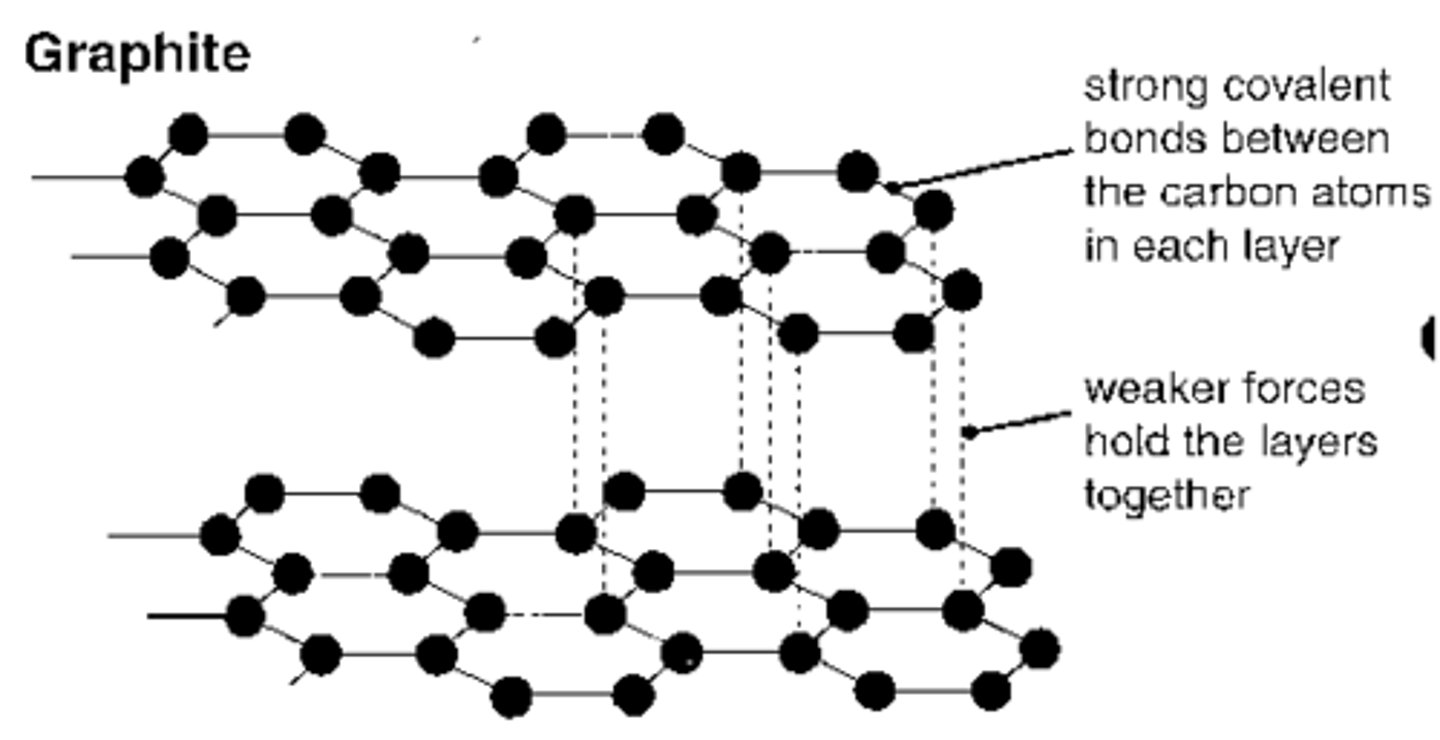
Why can only graphite conduct electricity?
Graphite (& graphene) atoms only make 3 covalent bonds so each carbon can have one delocalised electron which is mobile therefore can carry charge.
The weak intermolecular forces between each layer also mean the layers can slide over each other causing graphite o be slippery.
What are the main shapes of molecules?
- Tetrahedral
- Pyramidal
- Linear
- Non-linear
- Trigonal planar
- Octahedral
What effects the shape of a molecule?
How many electron bonding pairs the molecule has and how many lone pairs they have. Lone pairs are more repulsive then normal bonding pairs which effects the bond angle and so the shape.
Two lone-pairs have the most repulsion for each other.
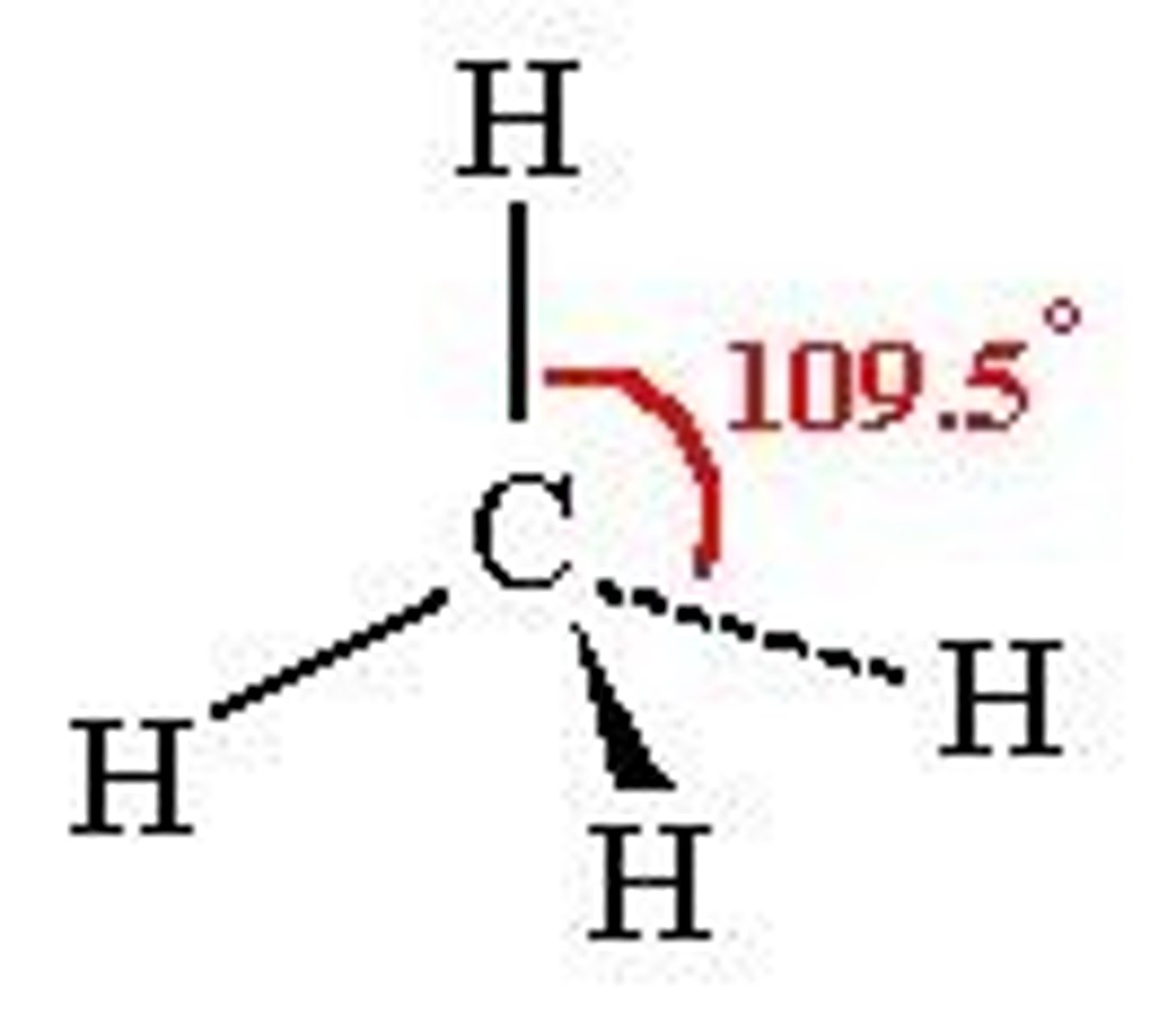
What shape is formed when there are 4 bonding pairs?
Tetrahedral :
- 4 bonding pairs
-e.g. Methane
- Bond angle of 109.5
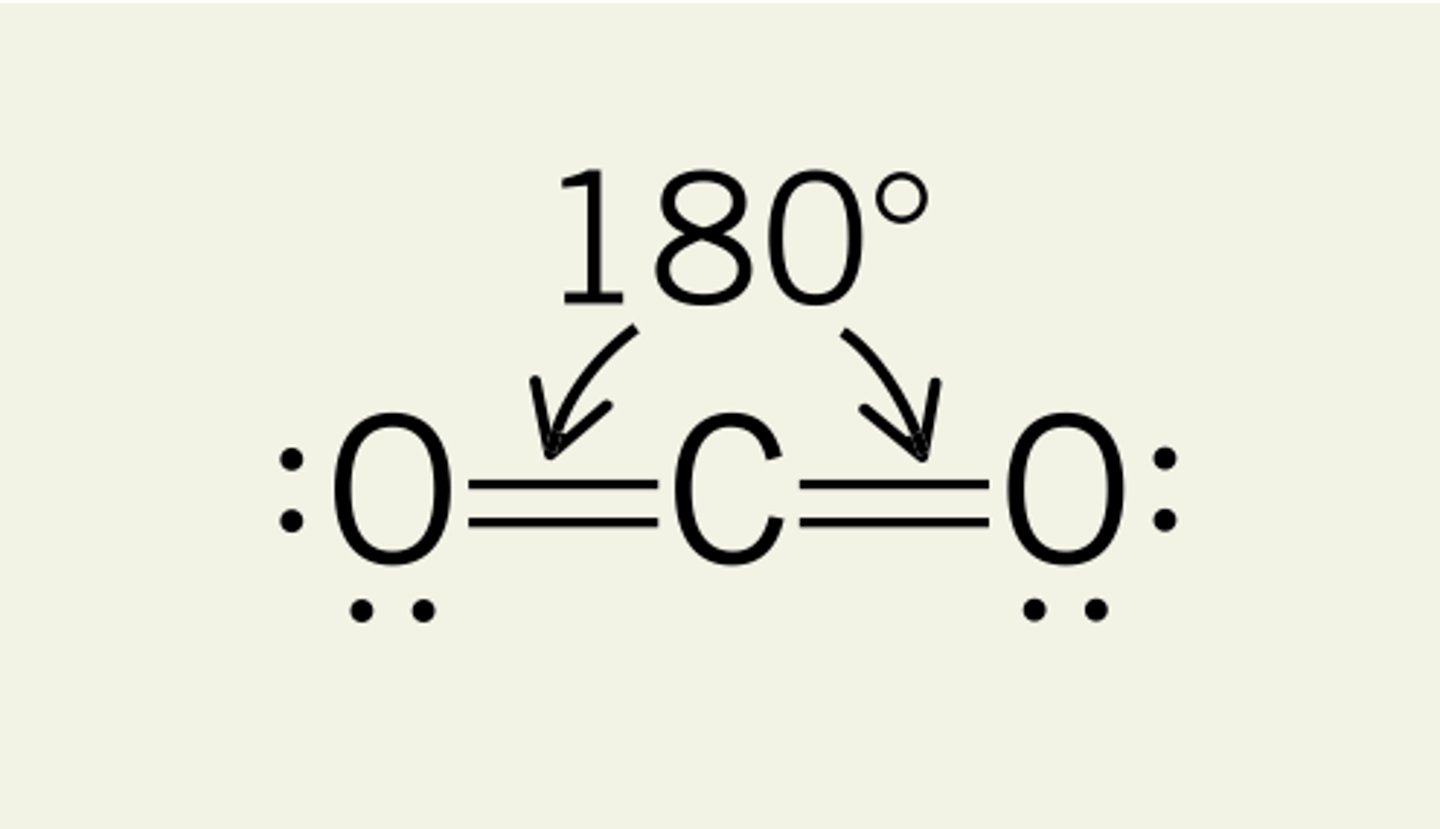
What shape is formed when there are 2 bonding pairs of electrons?
Linear :
- 2 bonding pairs
- e.g. Carbon Dioxide has a linear shape despite having a double bond as they don't effect shapes of molecules
- Bond angle of 180
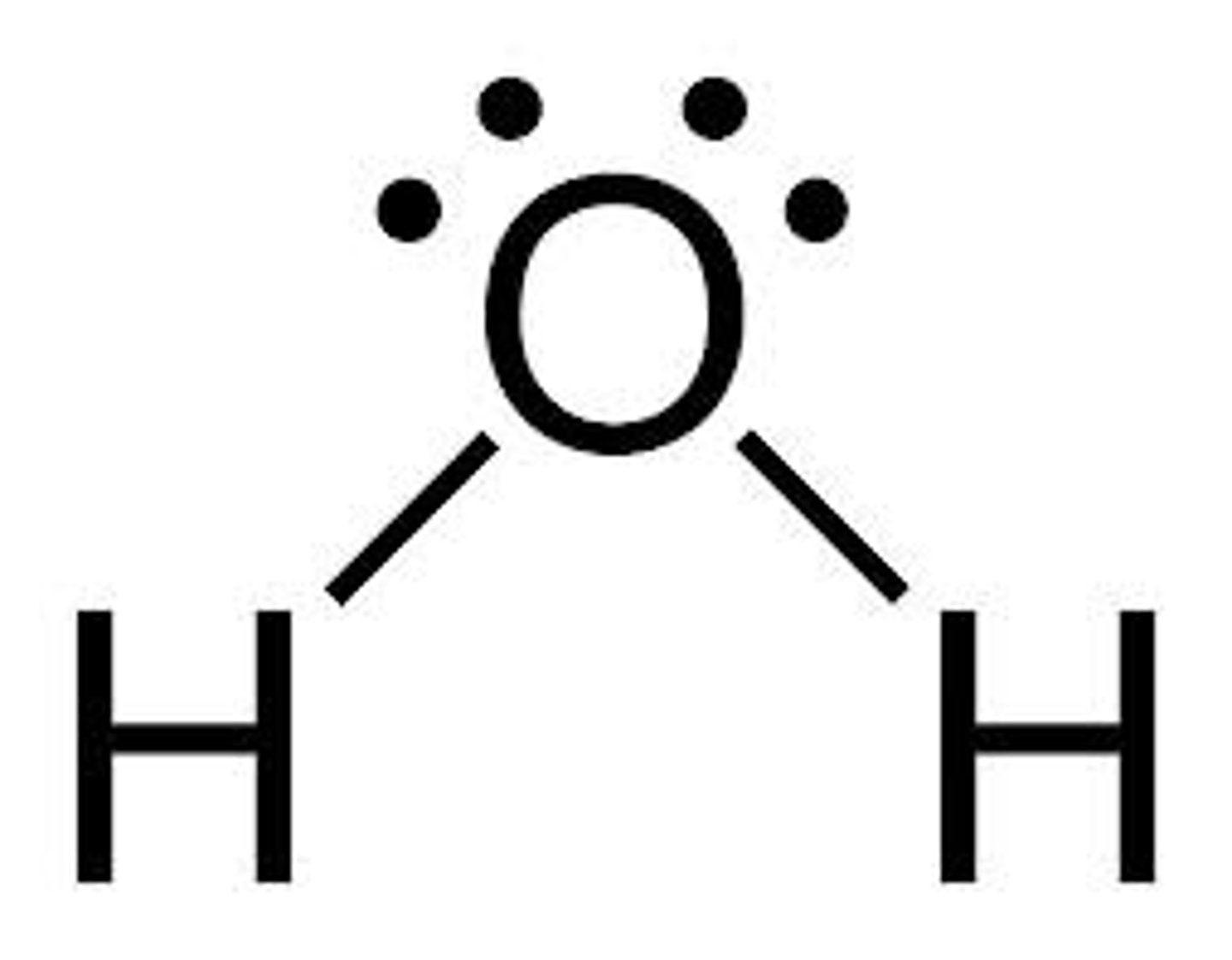
What shape is formed when there are 2 bonding pairs and 2 lone pairs?
Non-Linear :
- 2 bonding pairs & 2 lone pairs
- e.g H20
- Bond angle of 104.5
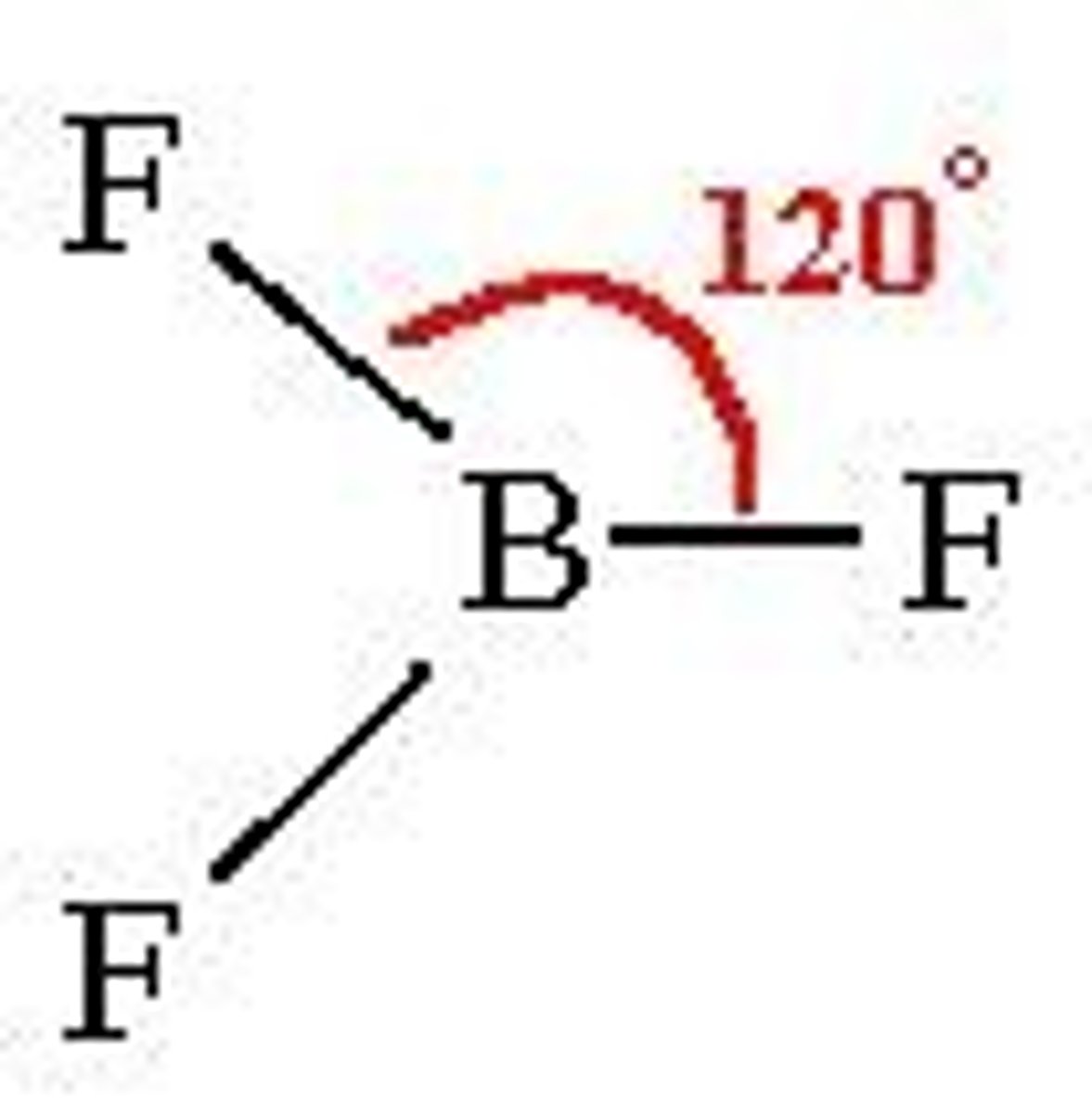
What shape is formed when there are 3 bonding pairs of electrons?
Trigonal Planar :
- 3 bonding pairs
- e.g Boron Trifluoride
- Bond angle of 120
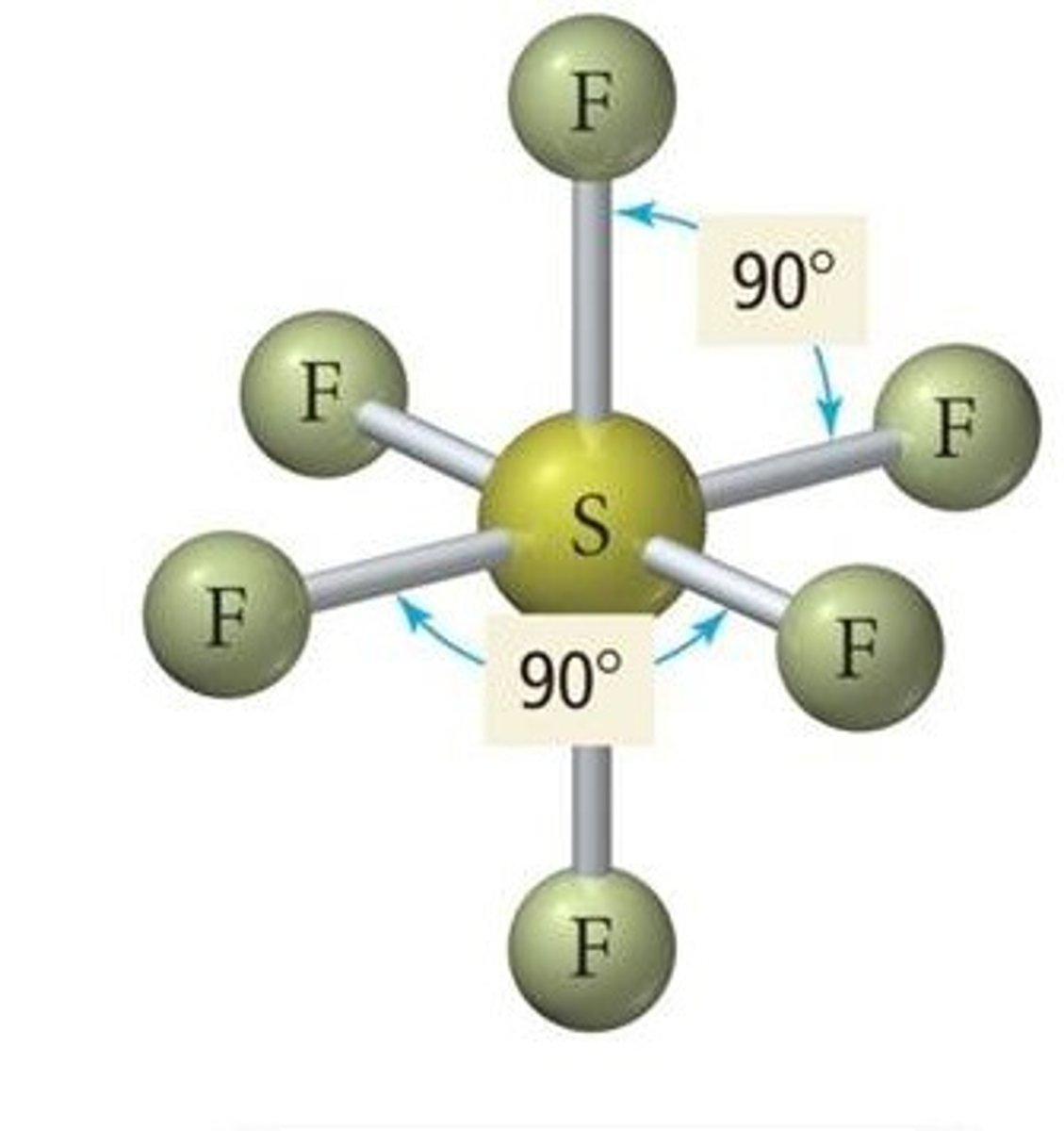
What shape is formed when there are 6 bonding pairs of electrons?
Octahedral:
- 6 bonding pairs
- e.g. Sulfur Hexafluoride
- Bond angle of 90
What is the pattern of bond angles when a lone pair is added?
When one lone pair is added 2.5 is taken of the bond angle.
E.g. tetrahedral to pyramidal = add one lone pair and:
109.5 - 2.5 = 107
What is electronegativity?
a measure of the tendency of an atom to attract a bonding pair of electrons in a covalent bond - therefore the electronegative an element is the more strongly it attracts a shared pair of electrons
What is the Pauling scale?
A measure of electronegativity that runs from 0 to 4. The greater the number the more electronegative the atom
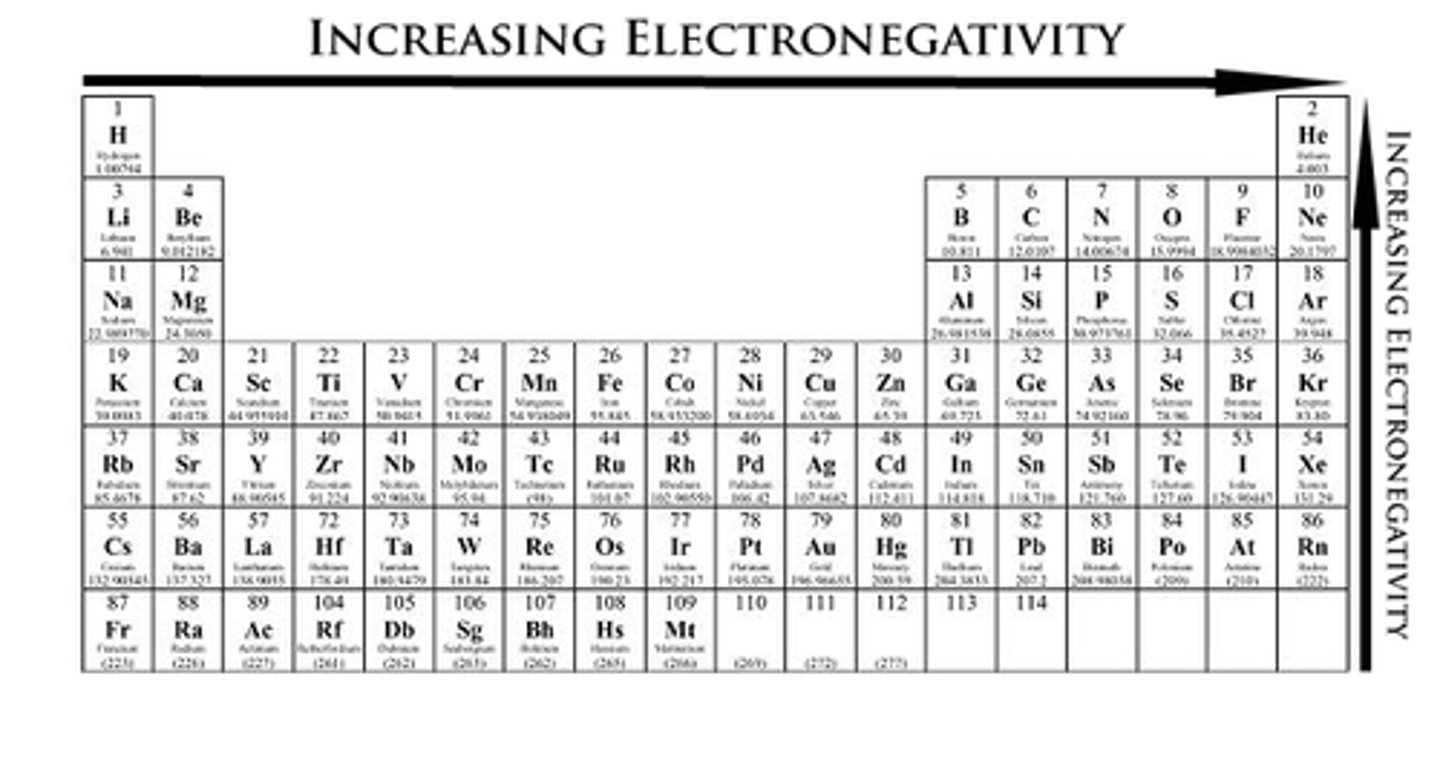
What is the pattern of electronegativity on the periodic table?
Non-metals = more electronegative with Fluorine as the most electronegative
Up a group or across a period = more electronegative
Why are some elements more electronegative than others?
- Atomic radius increases etc
- Nuclear charge
- Electron shielding
Why does electronegativity increase across a period?
It increases across because:
- Nuclear charge increases so there's a stronger nuclear attraction
- Atomic radius decreases because the nuclear attraction is so high even its own electrons are pulled towards the nucleus
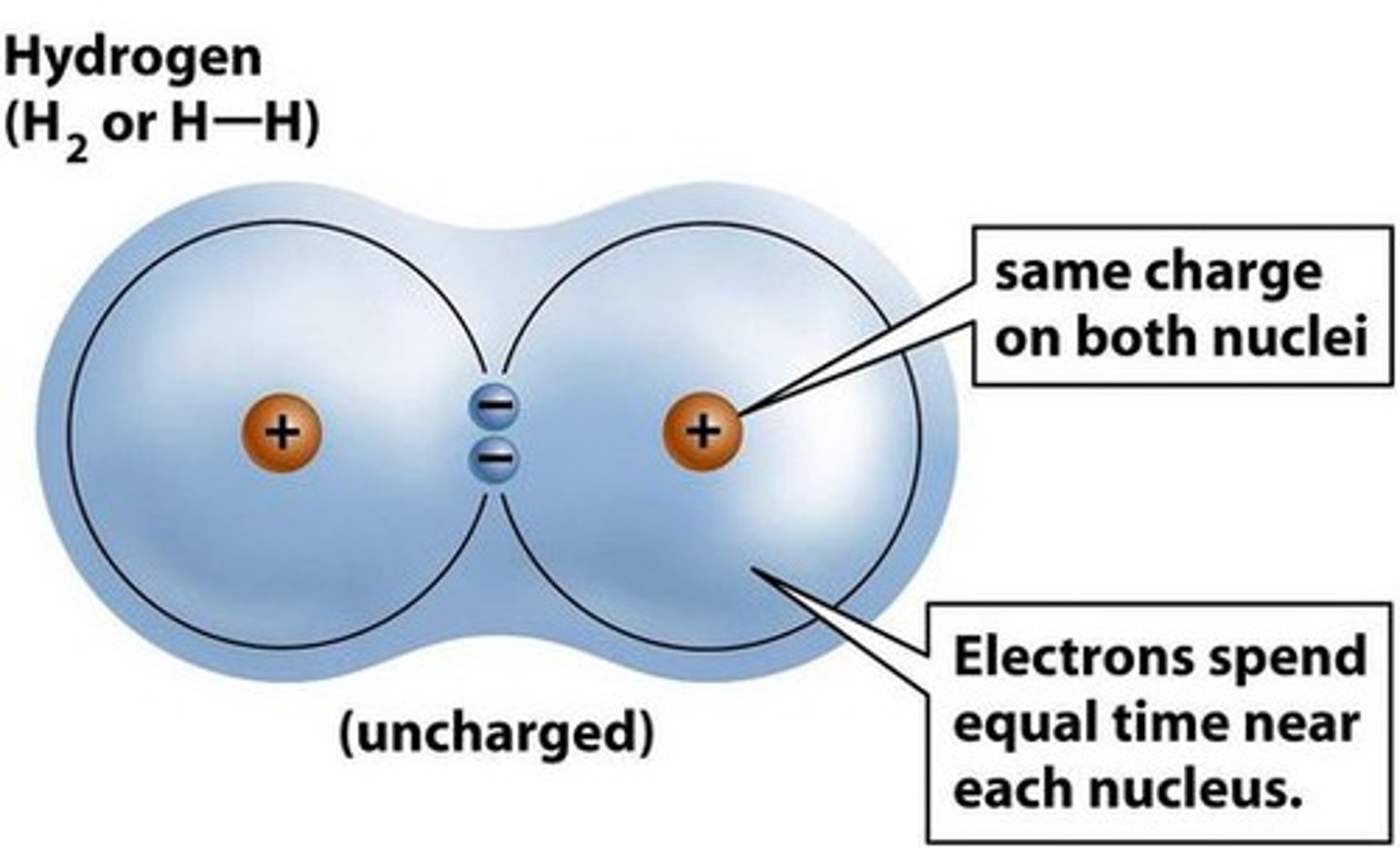
How are non polar covalent bonds formed?
The atoms are equally electronegative or as close as can be - same elements vs very similar elements.
So the bonding pair of elctrons are on average half-way between the 2 atoms therefore there's no large difference in electronegativity = non-polar bond.
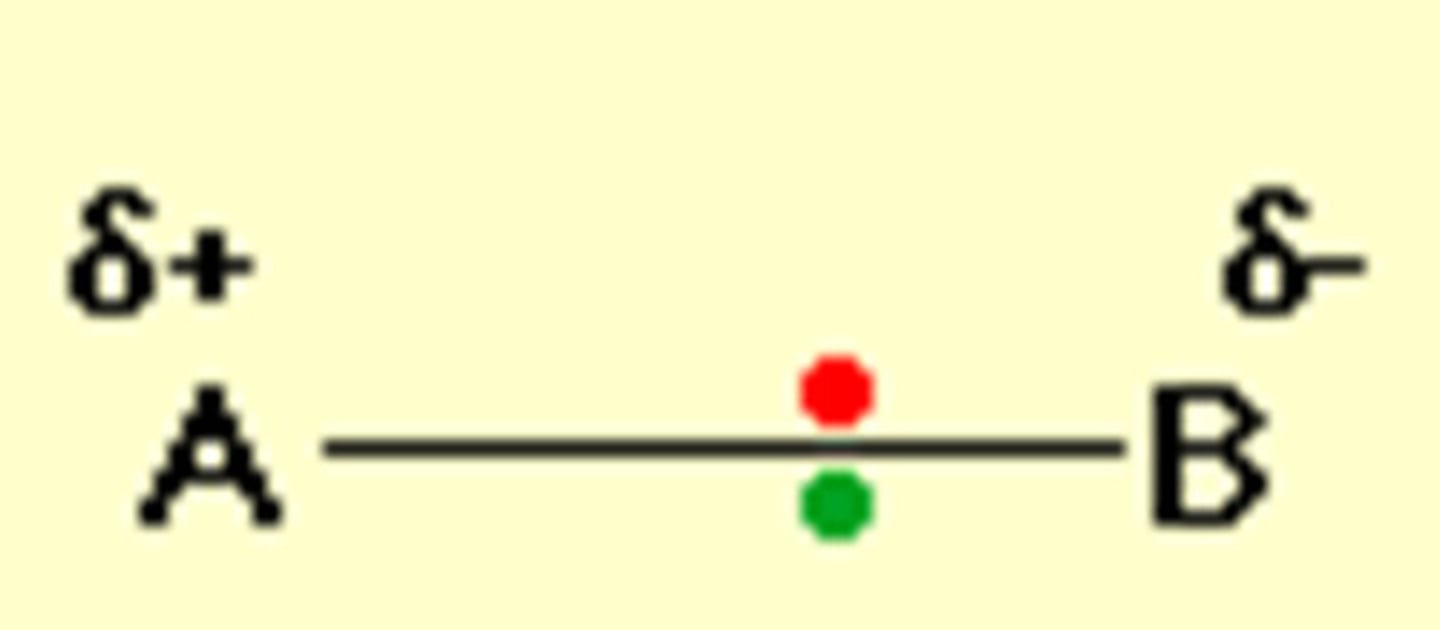
How are polar covalent bonds formed?
The atoms have significantly different elctronegativities .
So the bonding pair of electrons are shared unequally between the atoms so the atoms become slightly charged - not fully charged!
What are the 3 types of intermolecular forces?
Induced dipole-dipole interactions (London forces)
Permanent dipole-dipole interactions
Hydrogen bonding
What are induced dipole-dipole interactions/London forces?
Weak intermolecular forces that occur between all molecules - polar and non-polar. Specifically the act between induced dipoles in different molecules.
What is an instantaneous dipole?
A brief instance where an atom may have a slightly positive side and a slightly negative side due to the movement of electrons onto that side. This occurs because electrons are constantly moving.
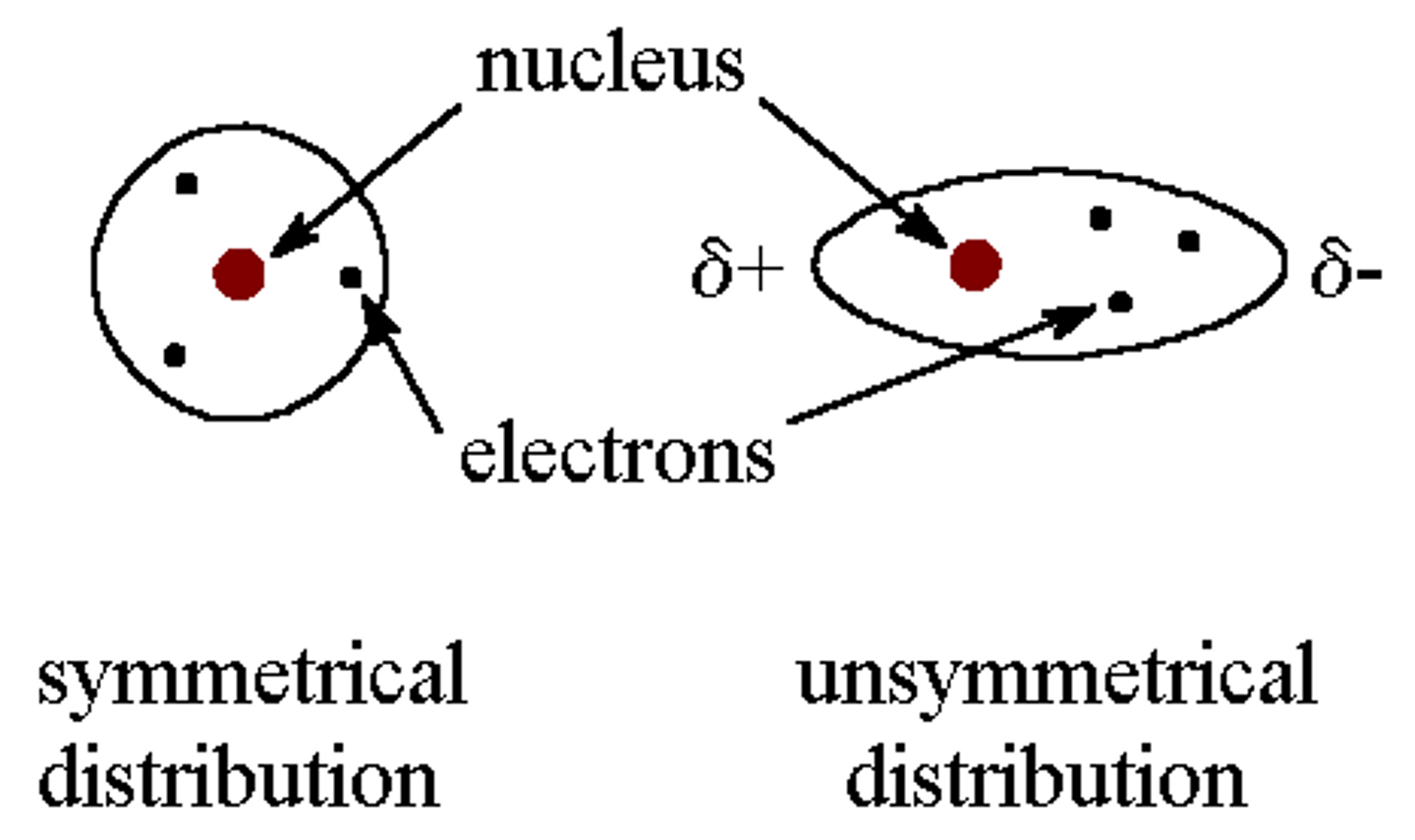
How do London forces occur?
An instantaneous dipole will induce a dipole on a neighboring molecule due to the slight charges on it causing a domino effect. London forces occur from interaction of all electrons between each molecules.
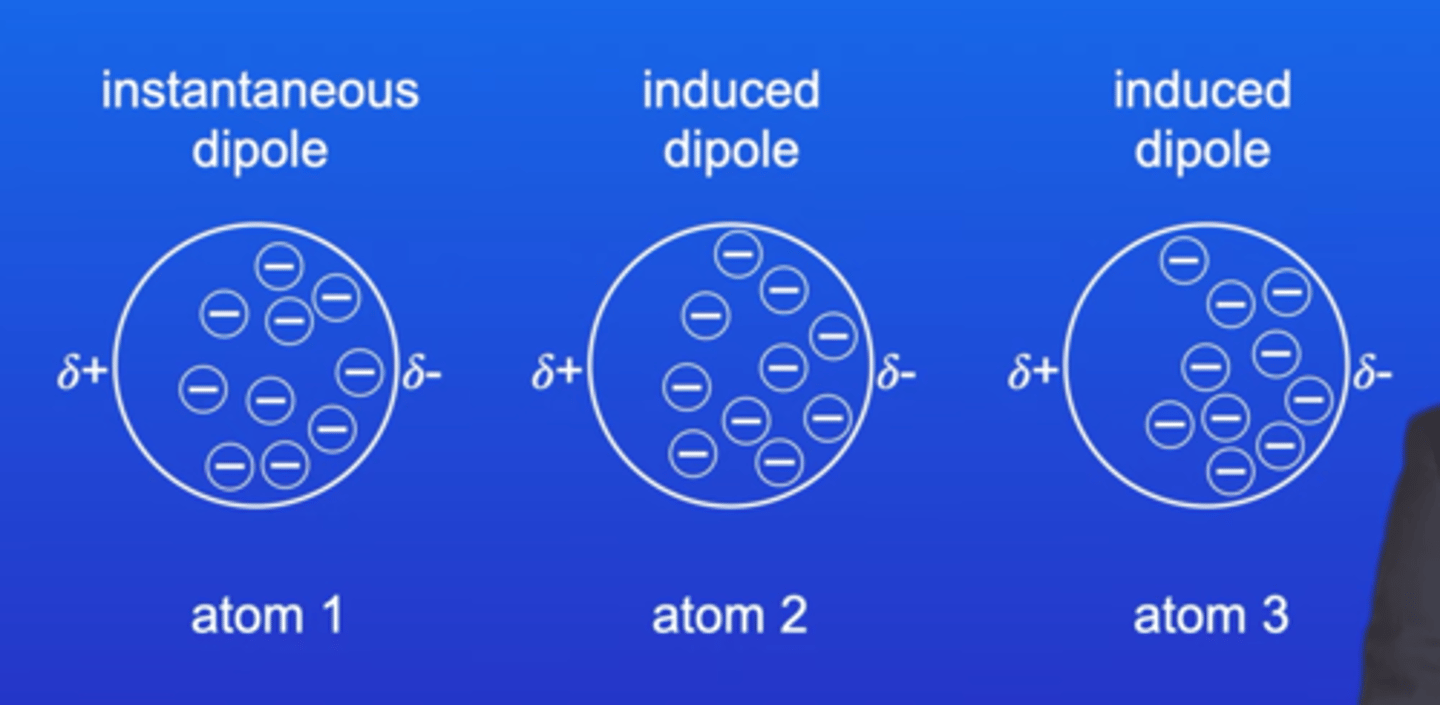
How can you increase the strength of a London force?
- Increase number of electrons in each molecule
- so stronger induced dipole-dipole interaction
- stronger attractive force
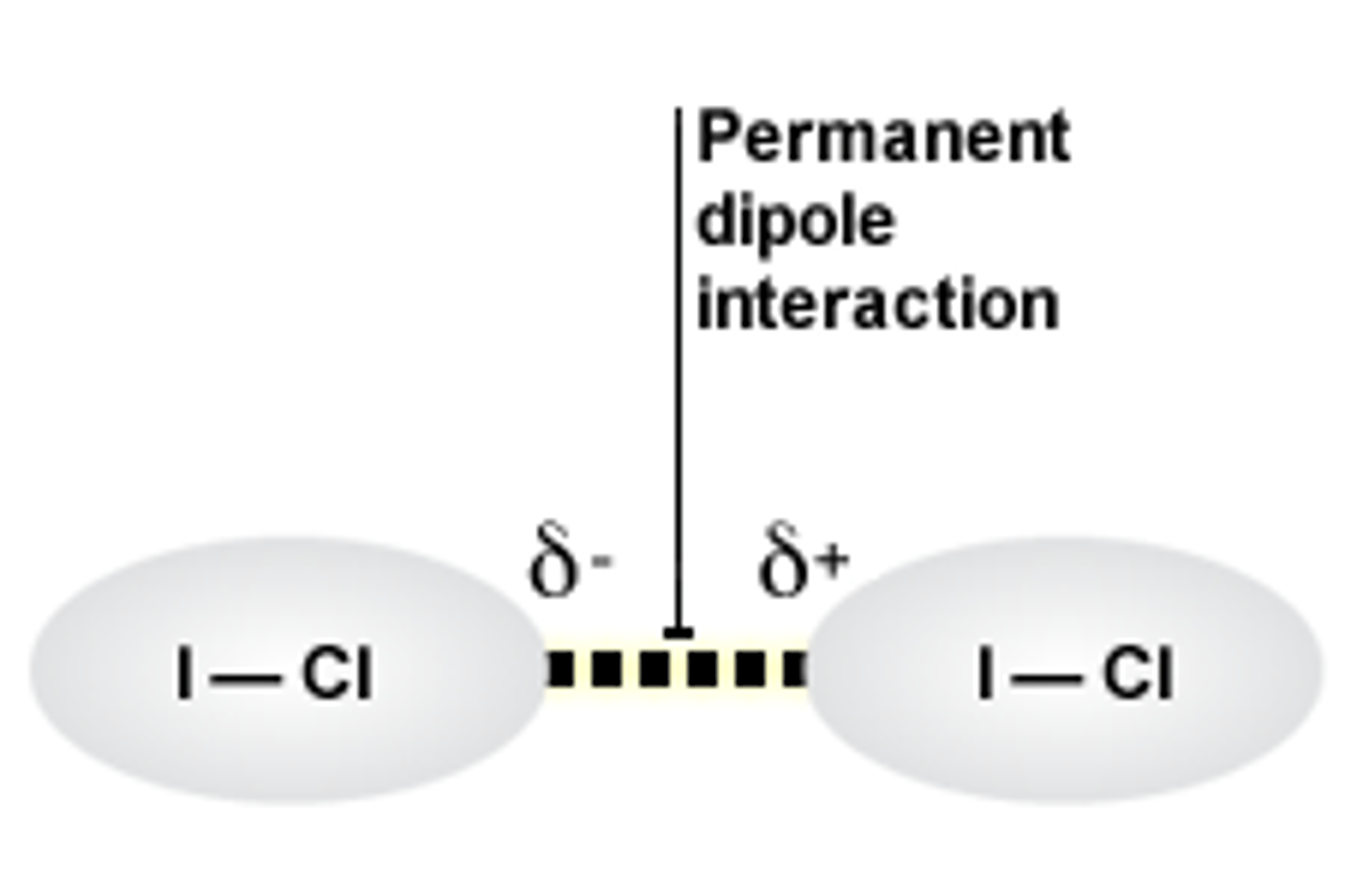
What is a permanent dipole-dipole interaction?
a weak attractive force between permanent dipoles and permanent dipoles so between polar molecules only - continuously occur as the dipoles are always slightly oppositely charged
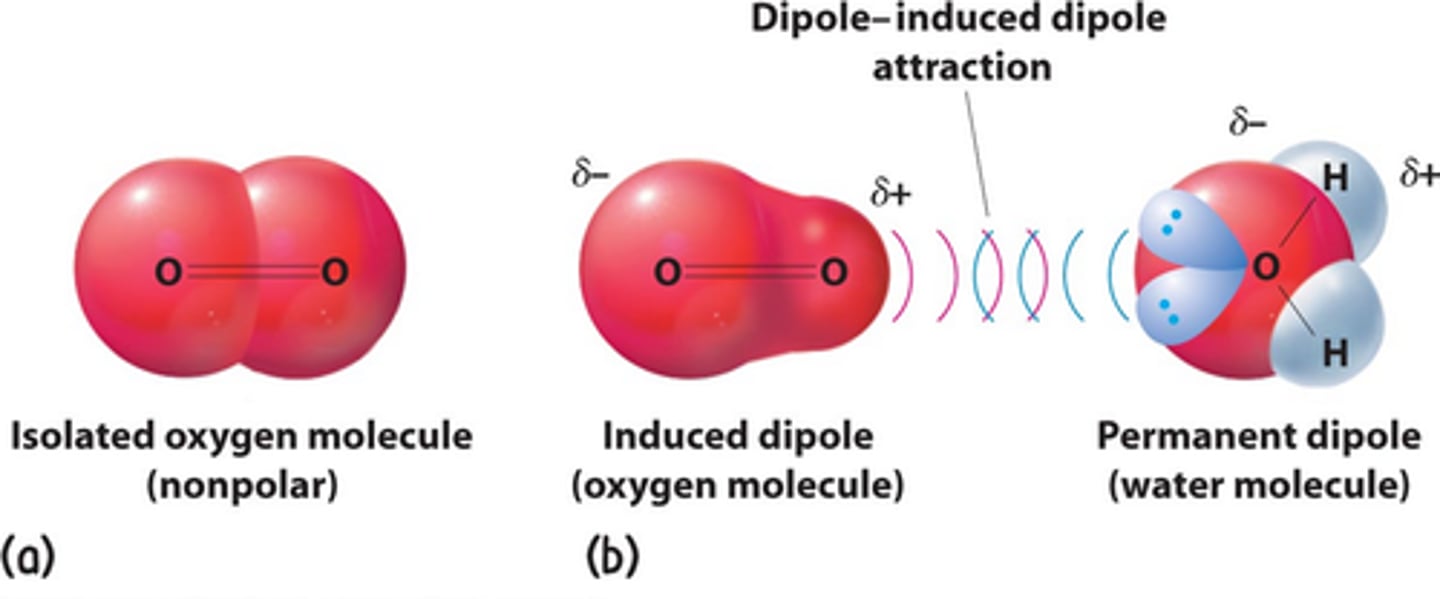
What is a permanent - induced dipole interaction?
A permanent dipole can induce a dipole in a non-polar molecule due to the difference in charge causing an attraction between them
E.g. HCl polar & Cl2 non-polar
Why are most non-polar molecules insoluble in polar solvents?
There is little interaction between the molecules and solvent so no induced dipole-dipole interactions are weakened etc causing most non-polar SMSs to be insoluble in polar solvent There are however exceptions.
How can some compounds dissolve in both polar and non-polar solvents?
They contain both polar and non-polar parts - however the longer/bigger the non-polar parts are the less soluble they are in polar solvents and vice versa.
What is a hydrogen bond?
A type of permanent dipole-dipole interaction found between molecules containing:
- an electronegative atom with a lone pair of electrons e.g. oxygen,nitrogen & fluorine
- a hydrogen atom attached to an electronegative atom
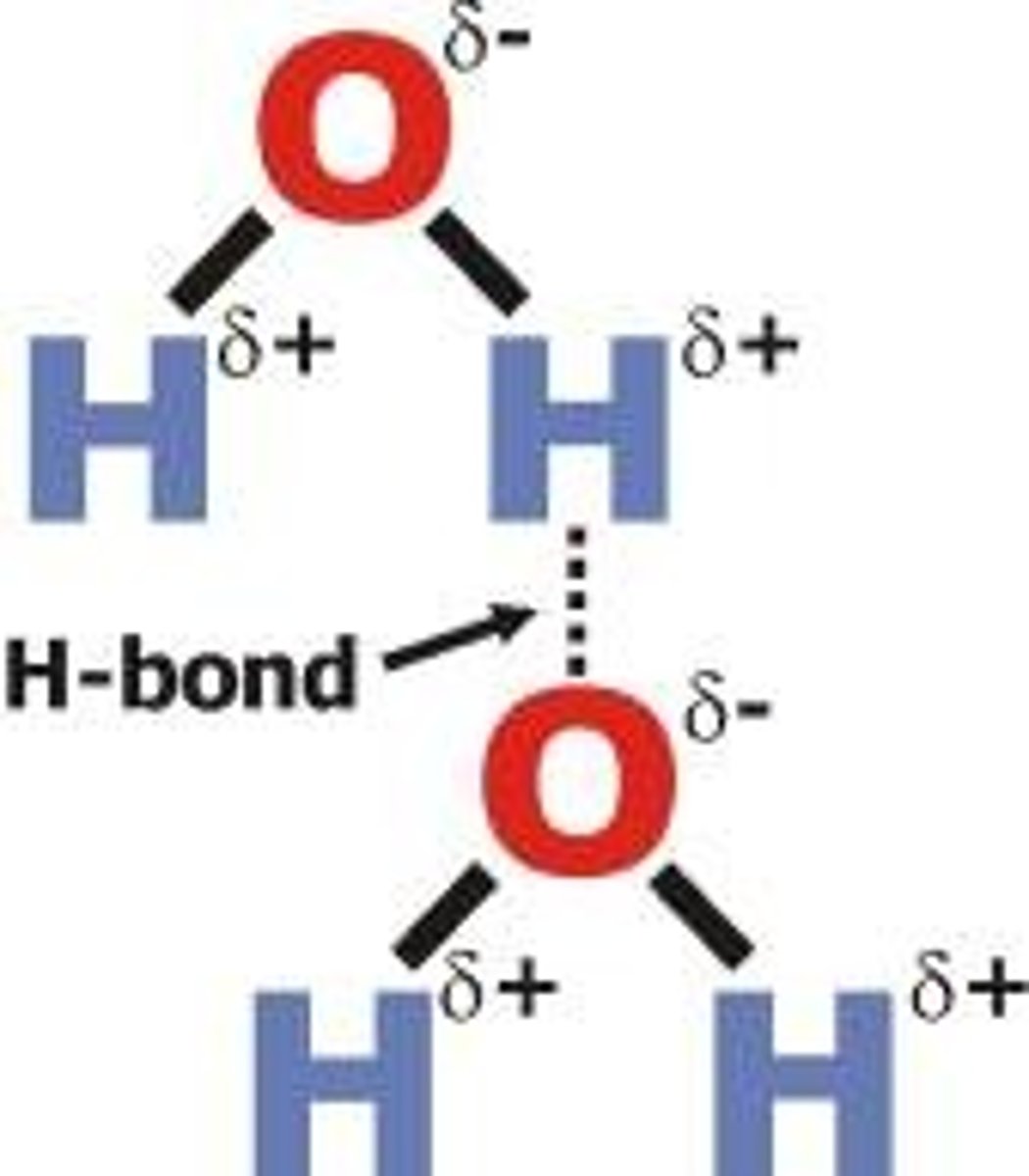
What does a hydrogen bond act between?
a lone pair of electrons on an electronegative atom in one molecule and a hydrogen atom in a different molecule
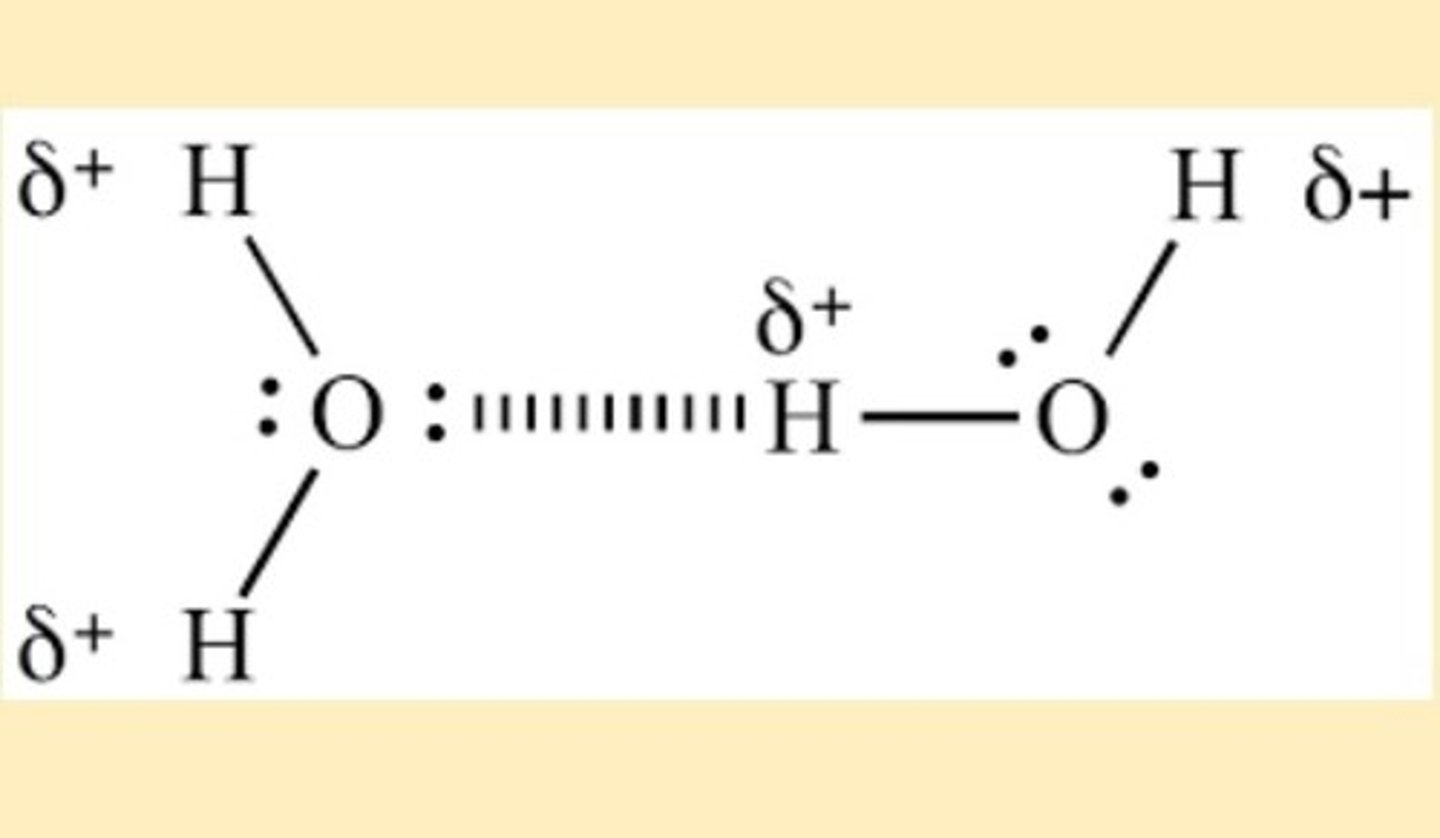
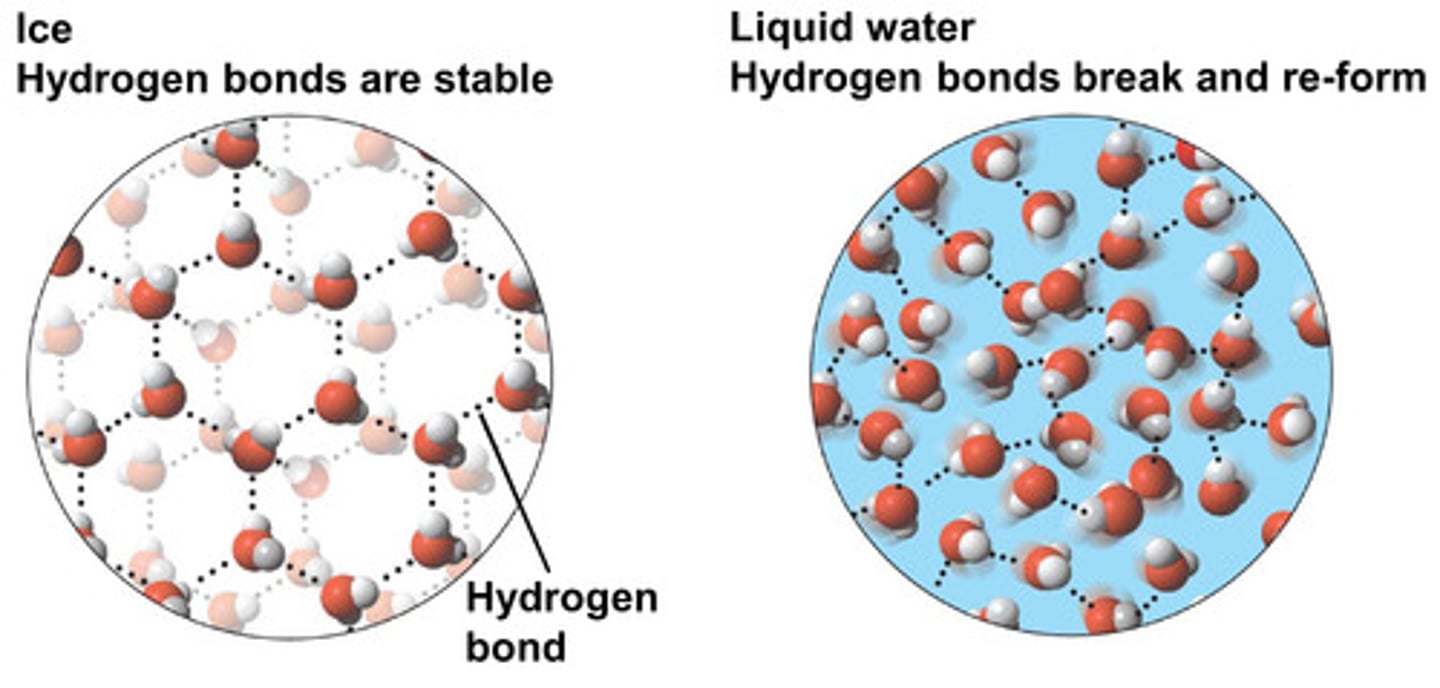
Why is a ice less dense than water?
The hydrogen bonds in ice hold water molecules apart in an open lattice structure so they're further apart in ice than in water where the water molecules can move closer together.
Why does water have a higher melting & boiling point than predicted?
It has both hydrogen bonds and induced dipole-dipole interactions between the molecules. Hydrogen bonds are harder to overcome than London forces (alone) so require more energy.
Without the hydrogen bonding water would have a BP of -75 which is why its higher than predicted.
What is an acid?
A H+/ proton donor - a substance which dissociates into H + ions when in solution.
What is the difference between a strong acid and a weak acid?
A strong acid completely dissociates into H+ ions in an aqueous solution.
A weak acid only partially dissociates into H+ ions.