Reaction Rates and Integrated Rate Laws
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Reaction Rate
Change in concentration over time for reactants/products.
Rate Equation
Rate = k[A]^x[B]^y, x and y experimentally determined.
Integrated Rate Law
Relates reactant concentration to time for reactions.
First Order Reaction
Rate depends linearly on concentration of one reactant.
Differential Rate Law
Describes how reaction rate changes with concentration.
Concentration at Time t
Amount of reactant remaining after time t.
Initial Concentration
Concentration of reactant at time t=0.
Natural Log Form
ln[A]t - ln[A]0 = -kt for first order.
![<p>ln[A]t - ln[A]0 = -kt for first order.</p>](https://knowt-user-attachments.s3.amazonaws.com/e4124dfd-a0af-4ba7-85c3-7bb5d67ae822.jpg)
Half-Life
Time required for half of reactant to react.
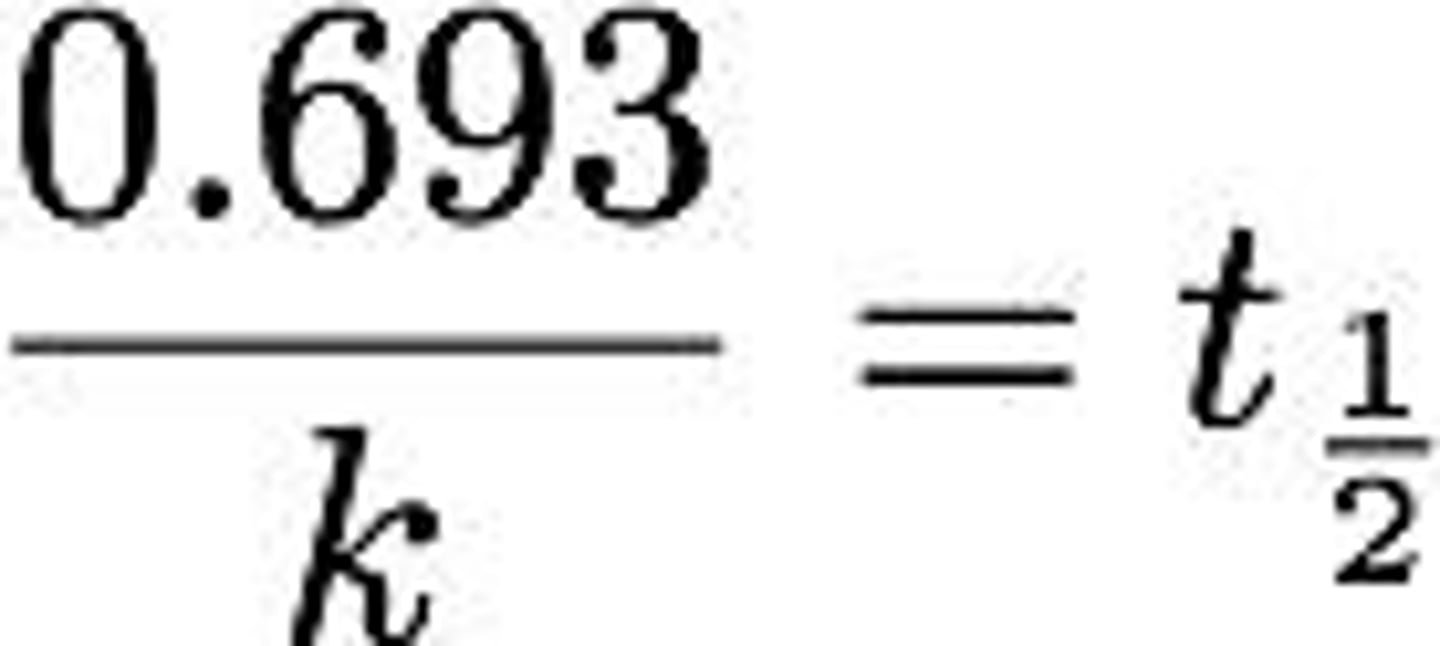
First-Order Half-Life
Independent of initial concentration [A]0.
Second-Order Reaction
Rate depends on square of concentration of one reactant.
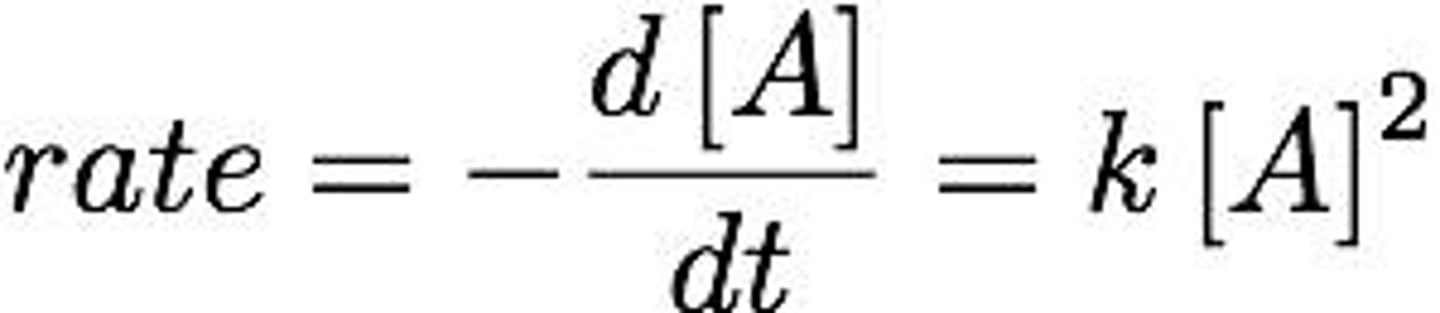
Second-Order Half-Life
Depends on initial concentration [A]0.
![<p>Depends on initial concentration [A]0.</p>](https://knowt-user-attachments.s3.amazonaws.com/9afe280a-857b-46b1-842d-d80f5b07bfa3.jpg)
Slope of First Order Plot
Slope = -k in ln[A]t vs. t plot.
Slope of Second Order Plot
Slope = k in 1/[A] vs. t plot.
Determining Reaction Order
Analyzing plots to identify reaction order type.
Data Collection
Experimental data used to determine reaction kinetics.
Kinetics
Study of reaction rates and mechanisms.
Stoichiometry
Relationship between reactants and products in reactions.
Concentration Terms
[A]0 and [A]t represent initial and current concentrations.
Graphical Analysis
Using plots to determine reaction order and rate.
Methyl Isonitrile Reaction
Example of a first-order reaction process.
NO2 Decomposition
Example used to determine reaction order through plotting.
Integrated Rate Law for Second Order
1/[A]t - 1/[A]0 = kt.
![<p>1/[A]t - 1/[A]0 = kt.</p>](https://knowt-user-attachments.s3.amazonaws.com/7d7f50a8-2eca-4c89-9e45-22d6c45d649a.jpg)
Rate Constant (k)
Proportionality constant in rate equations.