Summer - Nomenclature & Solubility
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
What are the diatomic elements?
BrINClHOF
Bromine
Iodine
Nitrogen
Chlorine
Hydrogen
Oxygen
Fluorine
(always have subscript of 2! ex) Br2)
What is ammonia?
NH3
a weak base
What are the carbon 1 to 10 alkanes?
methane (CH4)
ethane (C2H6)
propane (C3H8)
butane (C4H10)
pentane (C5H12)
hexane (C6H14)
heptane (C7H16)
octane (C8H18)
nonane (C9H20)
decane (C10H22)
C is just 1-10, H is C’s # x2 then +2 // C adds 1 while H adds 2 when going to the next one
What are the strong acids?
hydrobromic acid (HBr)
hydroiodic acid (HI)
hydrochloric acid (HCl)
nitric acid (HNO3)
perchloric acid (HClO4)
sulfuric acid (H2SO4)
all start with H
1st 3 hydro then Brickle (BrICl), last 3 Ni-perchloric-sulfur (Nitrate, Perchlorate, Sulfate (but H2 to cancel the ion))
What are the strong bases?
barium hydroxide (Ba(OH)2)
calcium hydroxide (Ca(OH)2)
strontium hydroxide (Sr(OH)2)
potassium hydroxide (KOH)
lithium hydroxide (LiOH)
sodium hydroxide (NaOH)
Baka Sr. Klina (BaCa Sr KLiNa), Baka Sr. has double OH
all end in ‘OH’ (hydroxide)
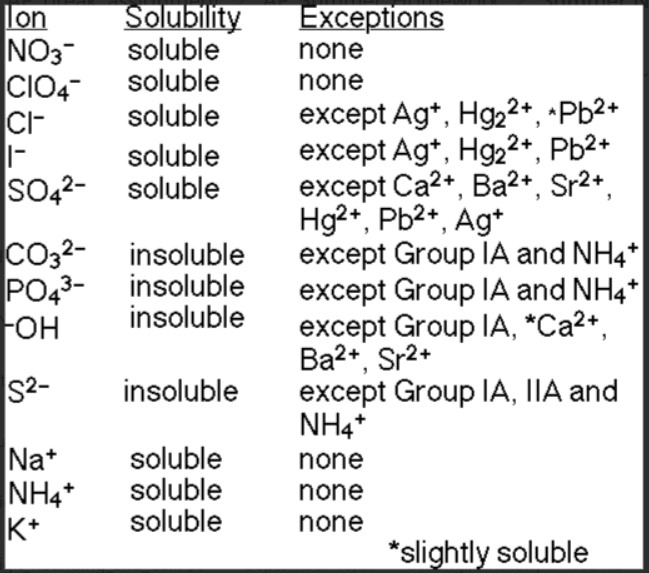
Solubility rules for ions
⭐Always soluble (PerNiA)
Nitrate (NO3-)
Perchlorate (ClO4-)
Ammonium (NH4+)
Sodium (Na+)
Potassium (K+) (also Group 1 elements (alkali metals))
✅Soluble BUT (Clisu - Clisu but silver/mercury/lead, su also but CaBaSr)
Chlorine (Cl-)
except Ag+, Hg22+, Pb2+ (slightly soluble)
Iodine (I-)
except Ag+, Hg22+, Pb2+
Sulfate (SO42-)
except Ca2+, Ba2+, Sr2+, Hg2+, Pb2+, Ag+
❌Insoluble BUT (CarboPhoSOH - CarboPhoS but Group 1A/ammonium, S also but 2A, OH Group 1A & CaBaSr)
Carbonate (CO32-)
except Group 1A and NH4+
Phosphate (PO43-)
except Group 1A and NH4+
Sulfur (S2-)
except Group 1A , 2A, and NH4+
Hydroxide (OH-)
except Group 1A, Ca2+ (slightly soluble), Ba2+, Sr2+