Carbonyl groups
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
37 Terms
A double bond O attached to a carbon which is also attached to S is called what?
A thio ester
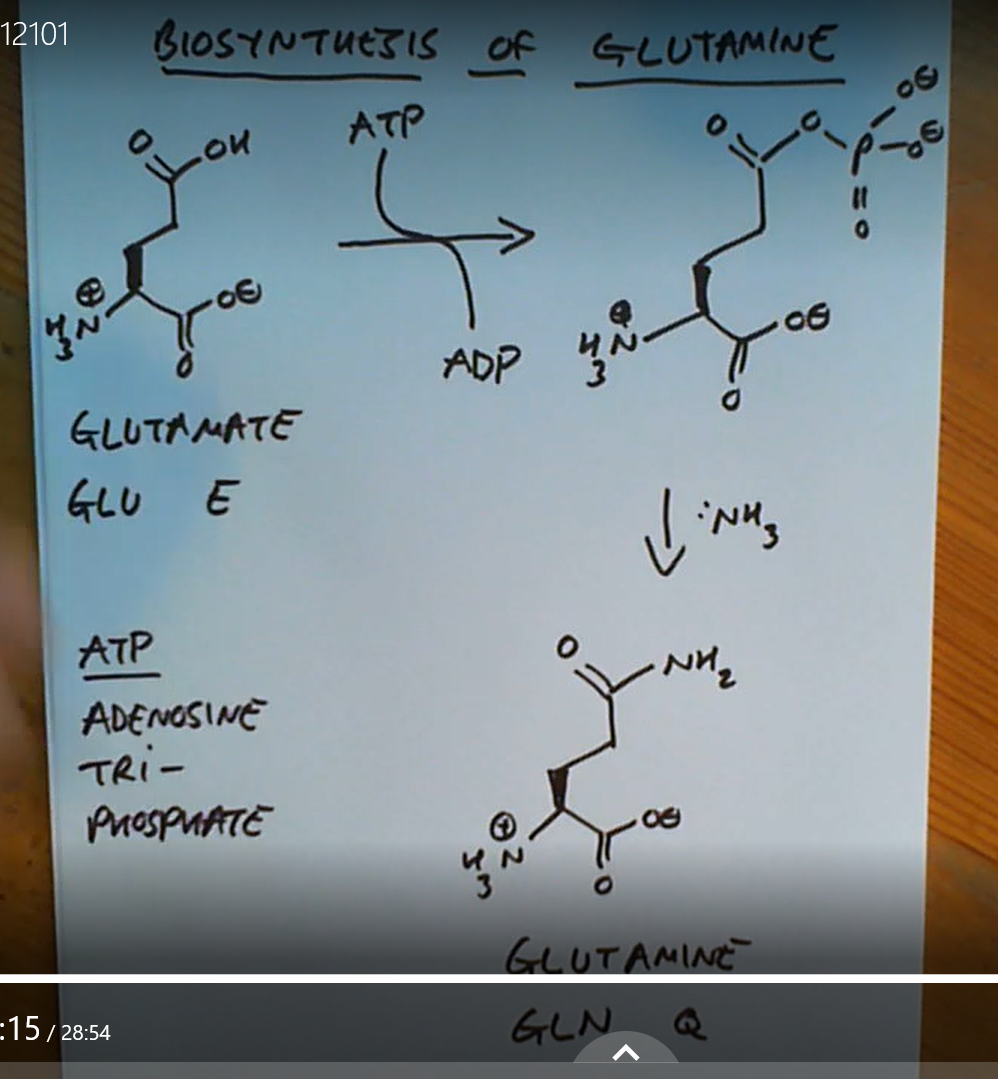
A double bond O attached to a carbon which is also attached to an N is called what?
An amide
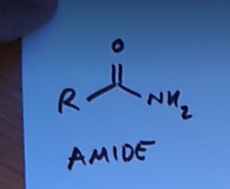
Two double bond O groups attached to a carbon with an O attached to the carbons inbetween them is a what?
An acid anyhride (means water is removed, two acids reacting with H2O being removed)
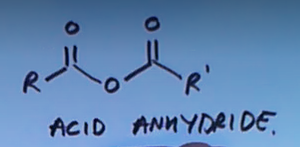
What determines carbonyl group chemistry?
The O is very electronegative so it pulls electrons towards it leading to it being delta minus therefore causing the carbon it’s attached to delta plus
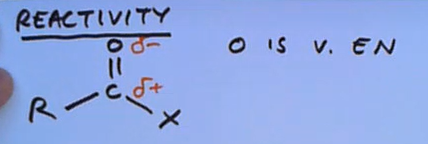
Because our carbonyl carbon is delta plus (an electrophile) what attack is it more susceptiable to?
Nucleophilic attack
When the nucleophile attacks the carbonyl carbon which bond always breaks first?
The pie bonds as they are much weaker compared to sigma bonds
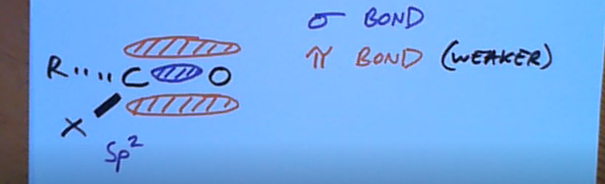
At alpha positions what’s special about the protons?
They’re acidic because electrons are pulled towards the oxygen
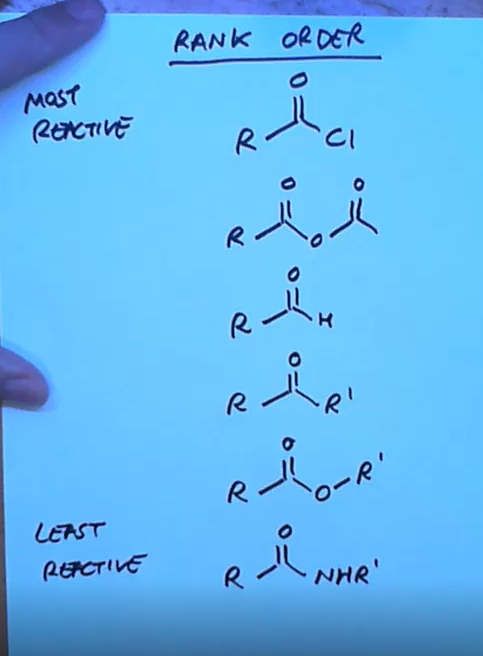
How do we rank order of carbonyl groups?
Based on leaving group ability and inductive effects
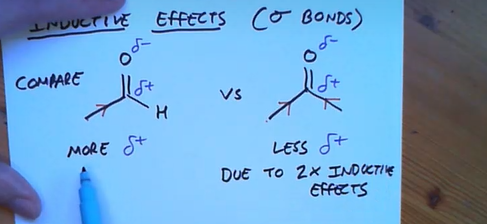
Why are aldehydes more reactive than ketones?
Because in ketone’s the inductive effect occurs twice making the carbonyl carbon less electrophilic
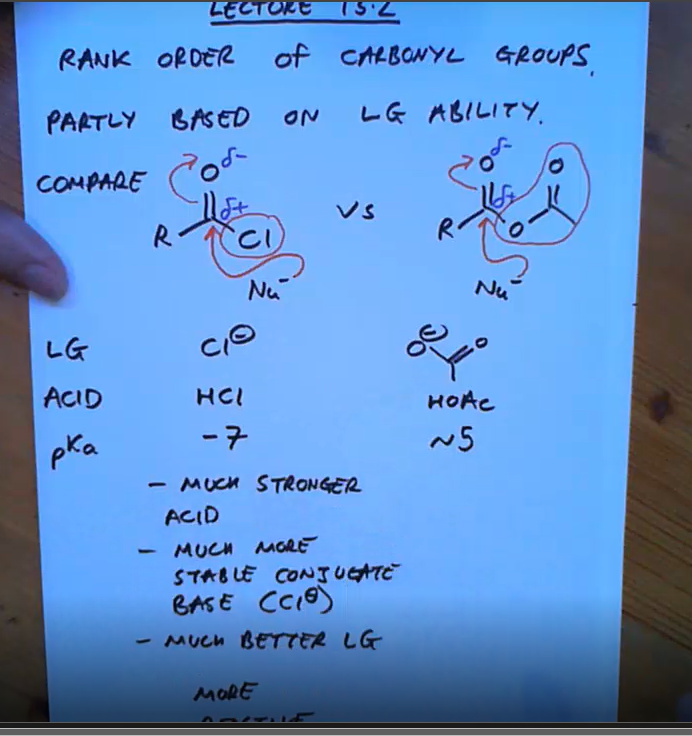
Why are acyl chlorides much stronger than acid anhydrides?
Because they are stronger acids so they are a better leaving group
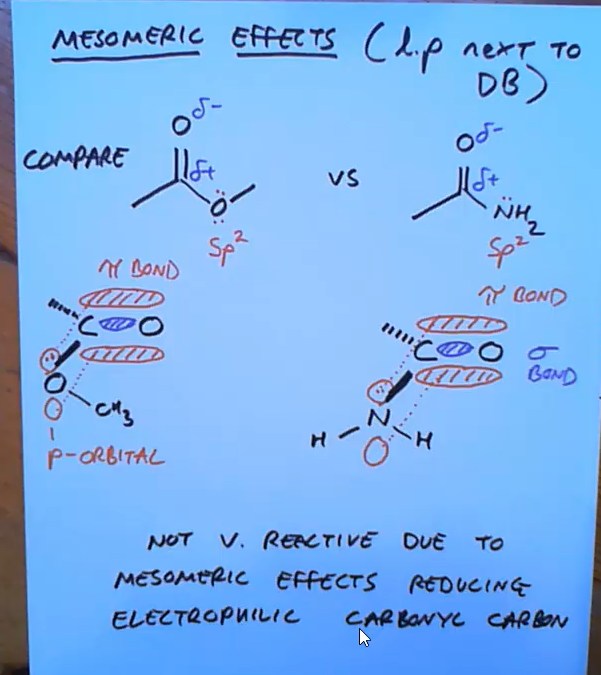
Why are esters more reactive than amides?
They’re both not that reactive due to mesomeric effect and pushing the elecrons onto the carbon but ester is more reactive than amide because oxygen is more electronegative
Why does nature use amide bonds?
As they’re not easily hydrolysed because they’re very stable
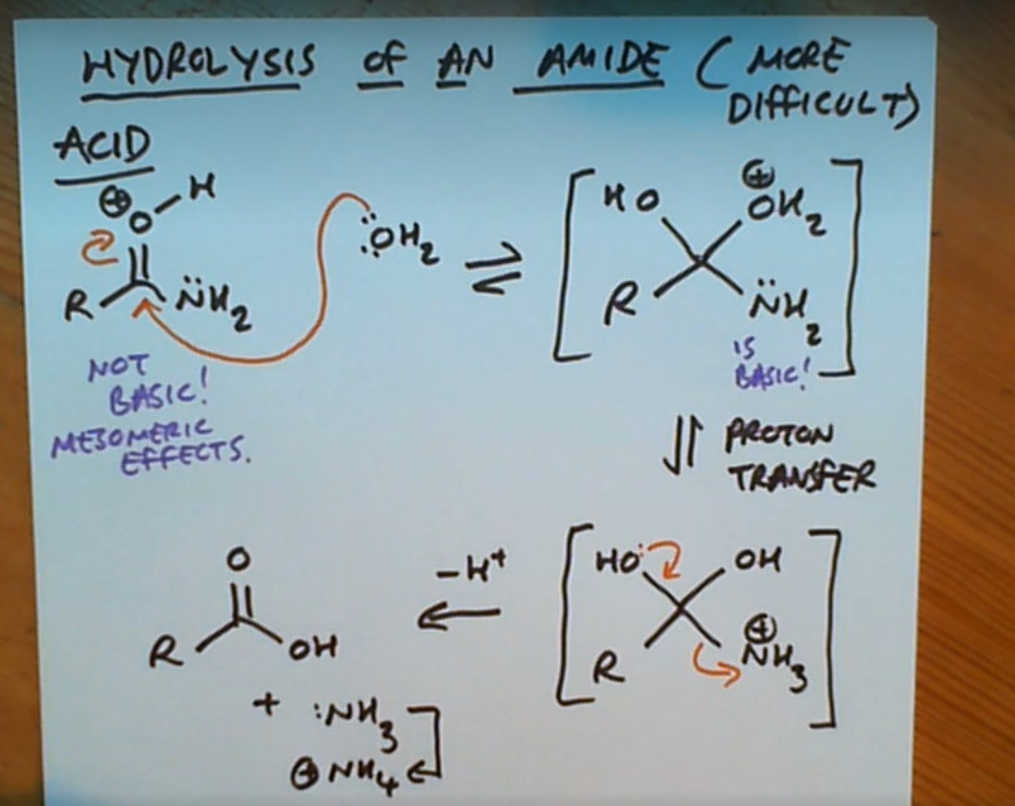
Why are amides not considered basic?
Due to mesomeric effects
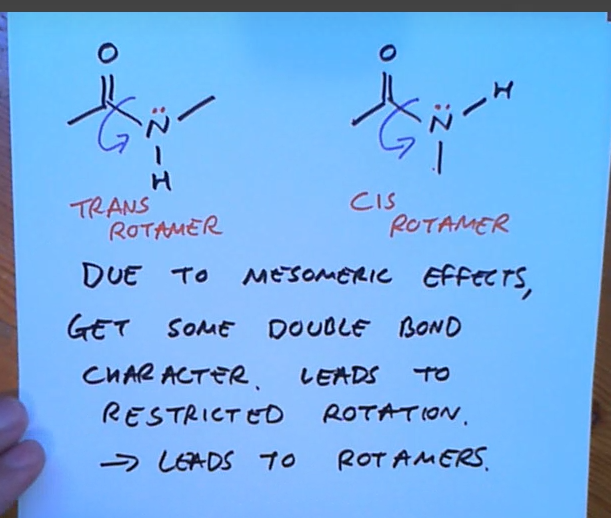
Mesomeric effects in amides can lead to what?
Rank order of carbonyl compounds
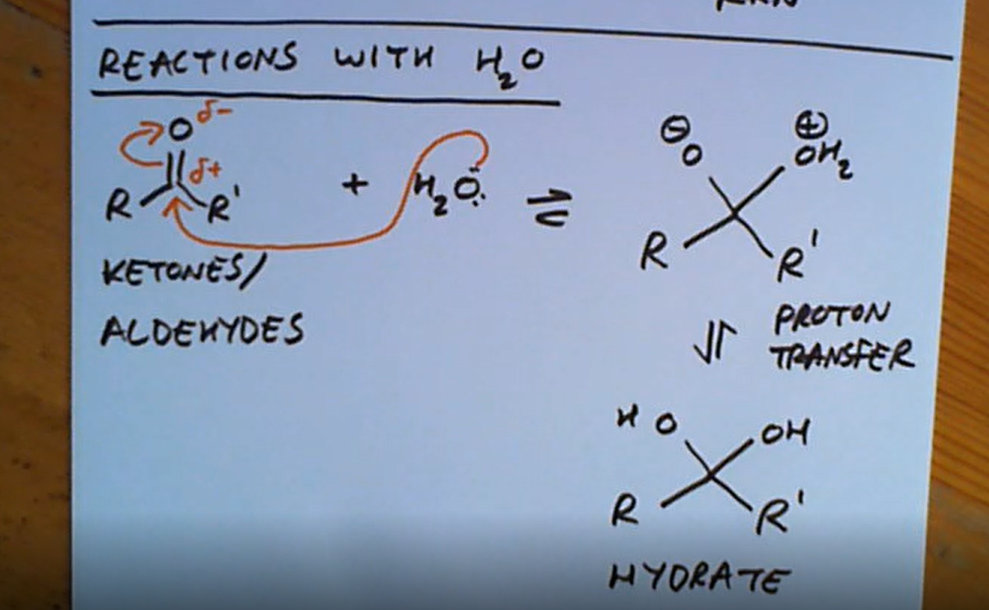
What are the two compounds which are carbonyl compounds that don’t have a leaving group?
Ketones and aldeydes
Ketones and aldehydes reaction with water
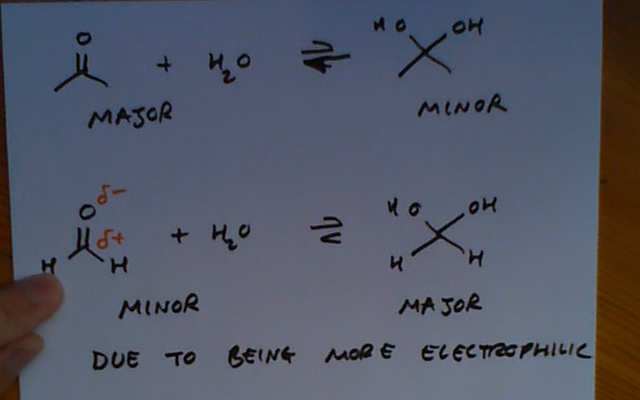
During aldehyde and ketone reactions which of the products and reactants are major/minor?
Usually the ketone/aldehyde is the major product and the hydrate is minor BUT when the ketone/aldehyde is VERY reactive due to inductive effect it is the other way round.
What’s another occurance where the hydrate is the major product rather than the orginal ketone/aldehyde?
Due to sterics, e.g for cyclo compounds bond angle’s are 60 constricting the sp2 hybridisation preventing it from being 120. 109 closer to 60 than 120!
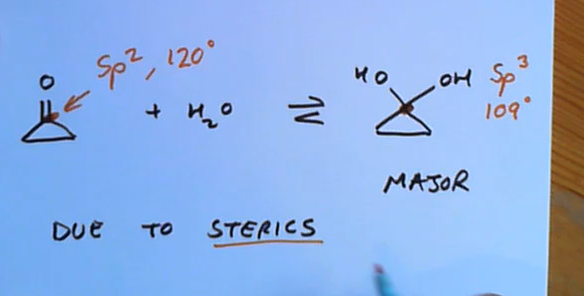
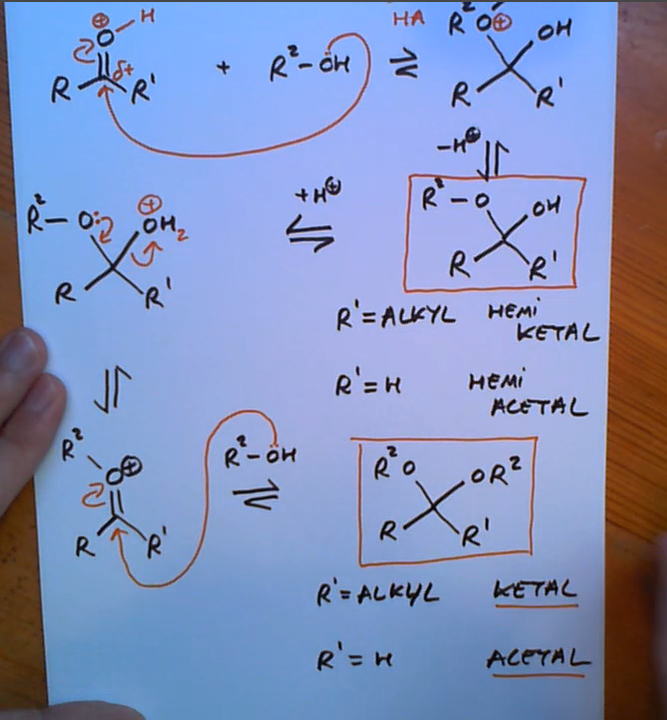
Ketone/aldehyde reacting with alcohol
Oxygen must be pronated first in acid catalysed carbonyl reactions
Ketone/aledhyde reaction with alcohol but within a chain
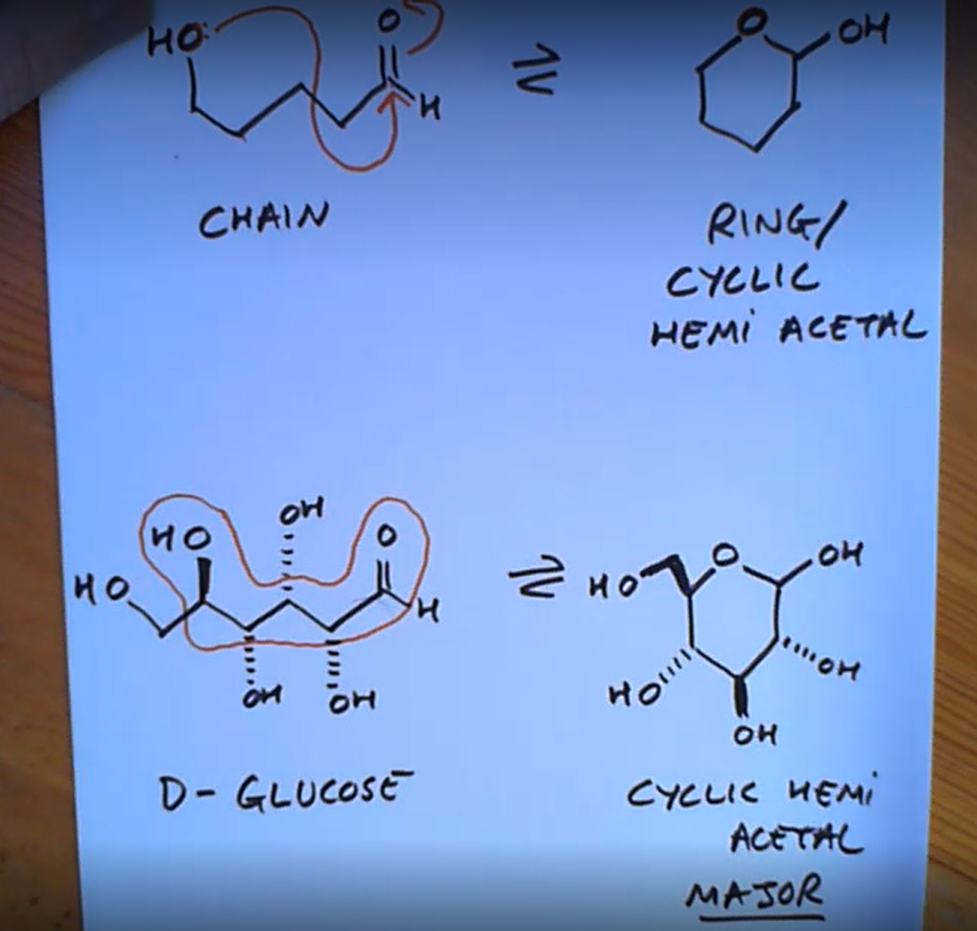
A squigly line in the chair conformation means?
Up or down so we have a pair of epimers
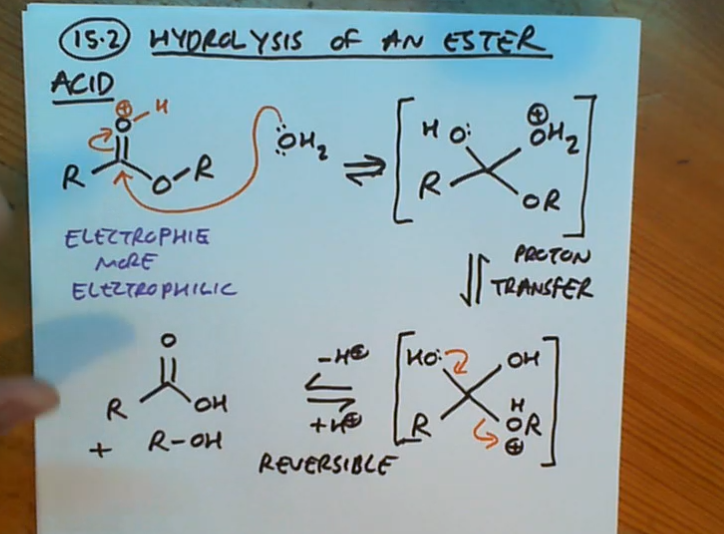
For the hydrolysis of an ester why do we use an acid?
Esters aren’t very reactive so the acid activates this by making the electrophile more electrophilic
How does the acid make our electrophile more electrophilic?
As it protnates the electrophile
Hydrolysis of an ester (acid)
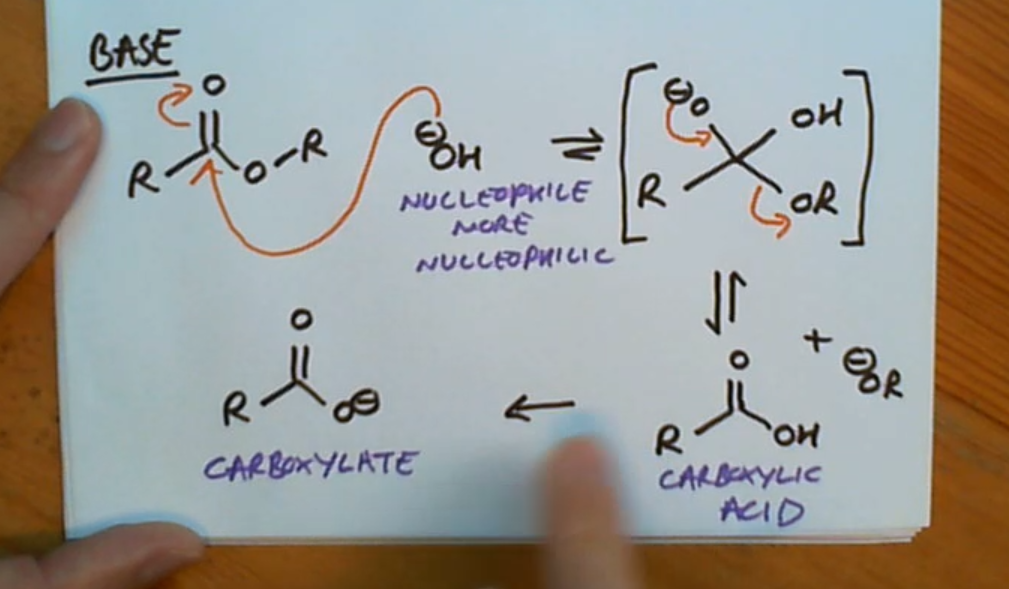
For hydrolysis of an ester via a base it makes our nucleophile..?
More nucleophilic
Hydrolysis of an ester (base)
Hydrolysis of an amide (acid)
Hydrolysis of an amide (base)
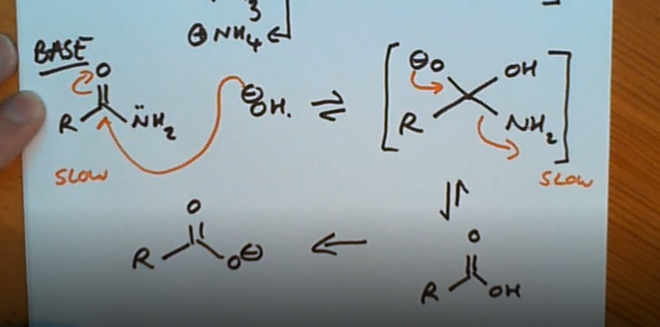
What’s special about hydrolysis using a base?
The carboxylate ion is formed at the end and is irreversiable
Forming an ester from a COOH and alcohol using an acid catalyst

Forming an ester from an acyl chloride with an alcohol
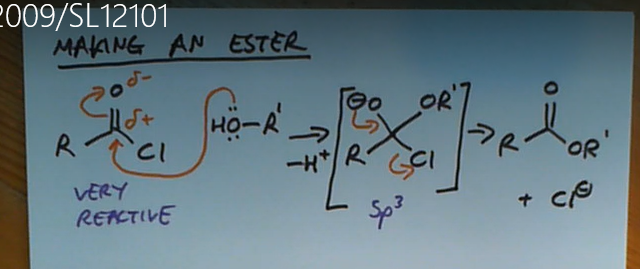
Forming an amide from an acyl chloride
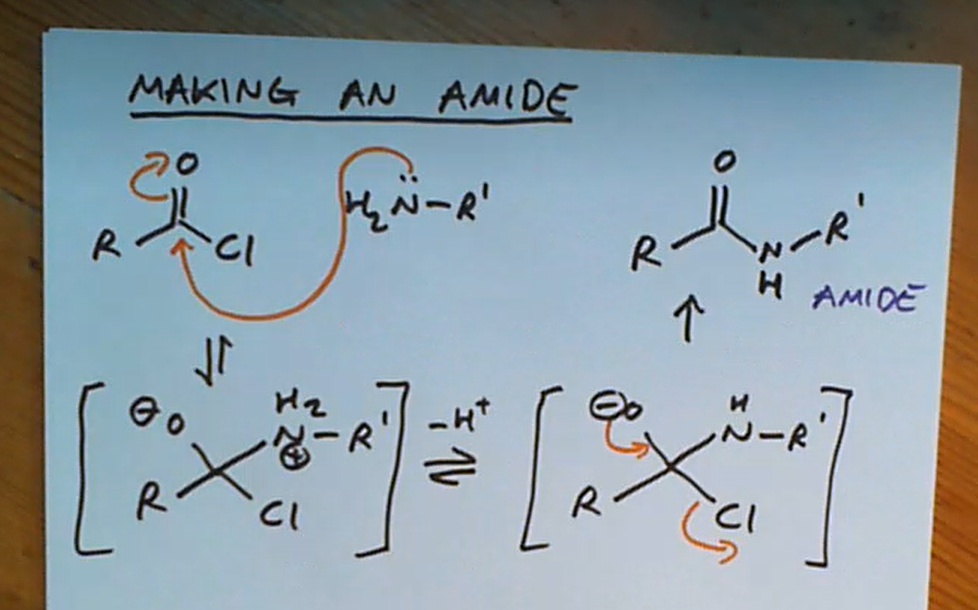
Can we form an amide from a COOH?
NO because an acid and base reacting forms a salt!
What 3 compounds does nature use to make amides from COOHs?
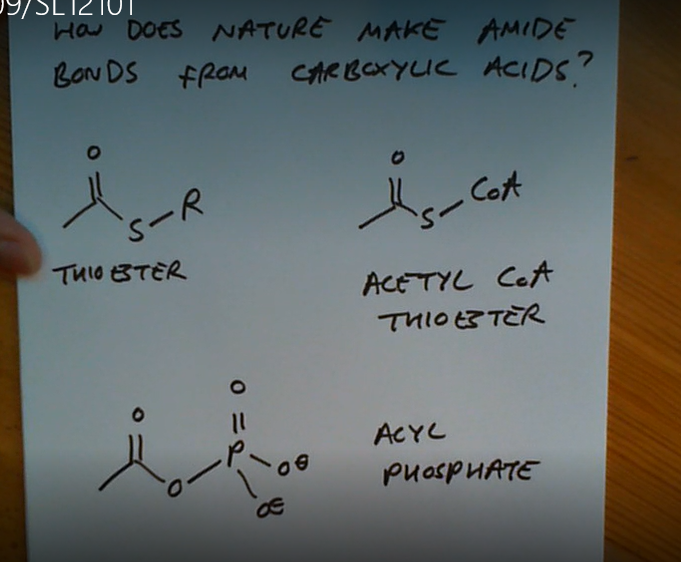
Why can’t nature use acyl chlorides?
Because they’re far too reactive
Biosynthesis of glutamine