Cell Injury
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
How does the heart enlarge? Cardiac muscle cells do not divide
cells need to enlarge: synthesize more cellular or cytosolic components
hypertrophy definition
increase in cell size
mechanism of hypertrophy (mechanical and trophic triggers)
stressor → stretch of myocytes or release of growth factors or hormones → signaling pathway to proteins
physiological vs pathological hypertrophy
physiological: stronger heart (good for you), during exercise
pathological: cell injury and progression to heart failure, 24/7
pathological hypertrophy
trying to make heart feel stronger, fetal gene expression
Hyperplasia definition
increase in cell number
what is required for hyperplasia
cells capable of dividing: less differentiated stem cells, differentiated cells
What two main ways cause physiologic hyperplasia?
Hormonal, compensatory
skeletal muscles can/cannot divide
smooth muscle can/cannot divide
skeletal cannot, smooth can
atrophy definition
shrinkage of cells from loss of substances
atrophy causes (3)
loss of stimulation (hormonal or nervous), inadequate blood supply, inadequate nutrients
main cause of ginigval hyperplasia
drug induced
what is autophagy? End result?
self eating, end result digest cellular components which provides nutrients for cell
why do cells atrophy?
small size enables survival
what 3 things integrate in autophage for nutrient breakdown?
sER membrane, autophagic vacuole, lysosome
metaplasia definition
switch from one cell type to another
metaplasia cancer relationship
metaplasia increases risk of cancer
3 ways of hypoxia
ischemia (reduced blood supply), reduced O2 carrying capacity, reduced oxygenation
two morphological pattern with reversible injury:
swelling, fatty changes (seen more often in liver)
when does cell swelling occur? what happens?
damage to plasma membrane or decrease in intracellular ATP
increase in ions (esp sodium), brings in more water
5 typical changes with reversible cell injury in swelling
blebls/loss of microvilli
ER swelling (vacuoles)
Mitochondria swelling
ribosome detachment (protein synthesis decreases)
nuclear chromatin clumping
gross changes with reversible cellular injury (swelling)
increased weight and turgor
increased pallor (compression of capillaries)
cellular changes with necrosis (4) (and consequences)
damaged lysosomal membrane (ROS + enzymes leaks out) → digest organelles
DNA damaged
damaged plasma membrane (cell contents leaks out, cell fragments)
mitochondrial damage (deplete ATP and generate ROS)
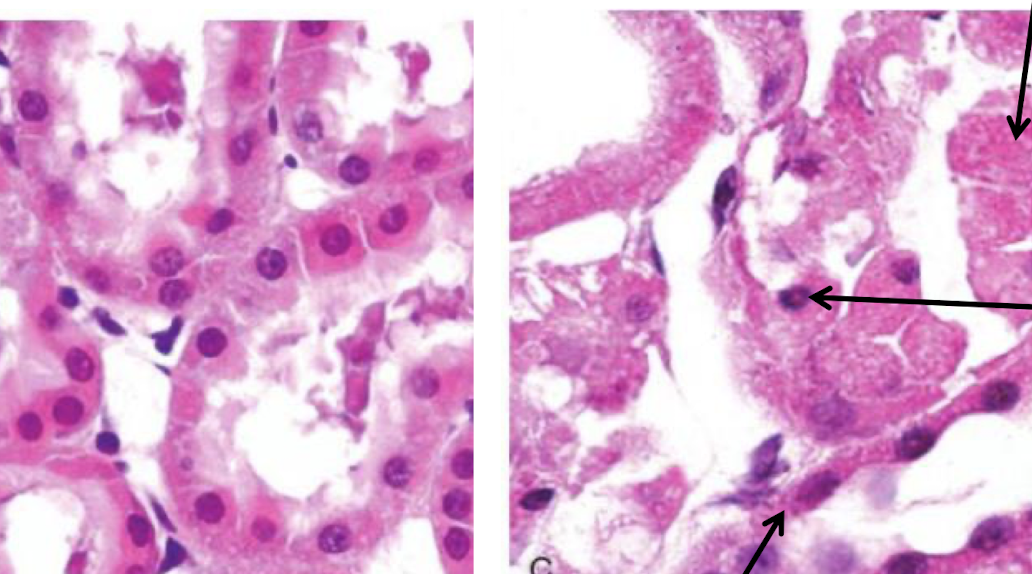
what happened?
necrosis
How does body respond to necrosis?
release of cellular contents → initiates inflammation
DNA condenses: becomes solid, shrunken, dark mass
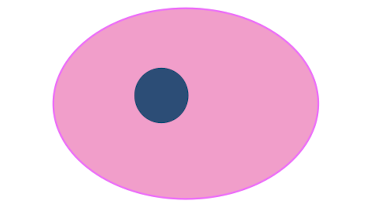
pyknosis
pynkotic nucleus fragments
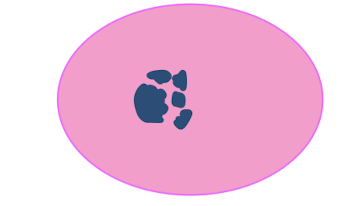
karyorrhexis
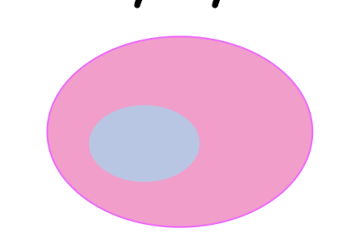
DNAase activation → DNA dissolution
karyolysis
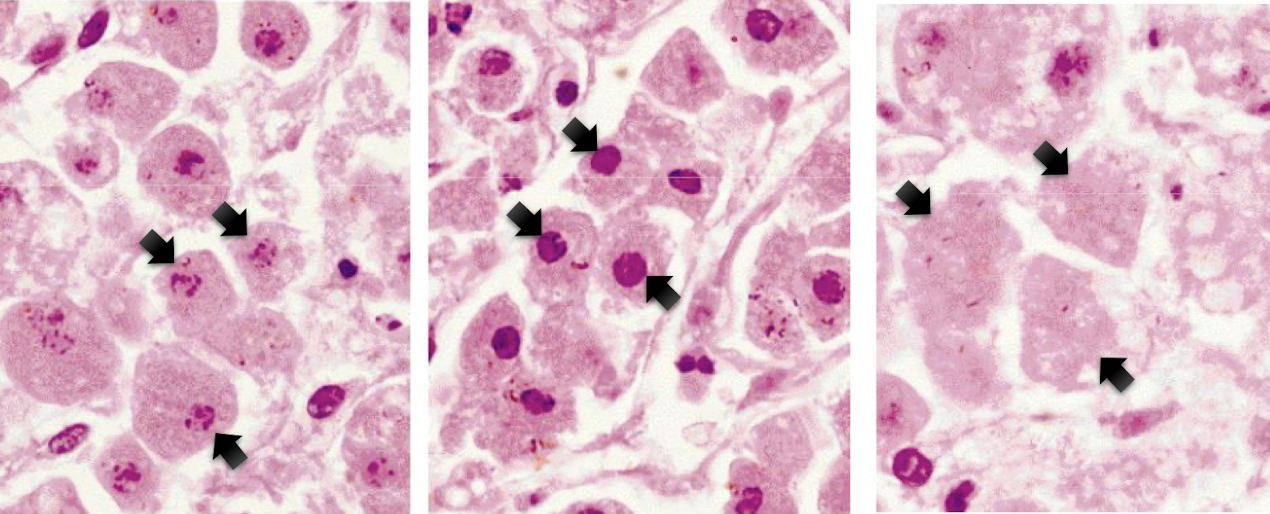
label each from left to right
karyorrhexis, pynkosis, karyolysis
coagulative necrosis often occurs in __ _?
solid organs (kidney)
main cause of coagulative necrosis
ischemia (loss of blood supply)
Coagulative necrosis color, texture, shape?
pale (no blood), firm (tissues not degraded), wedge shape
liquefactive necrosis main cause
focal bacterial or fungal infection → pus/proleytic enzymes that destroy surrounding tissue
an abscess is indicative of what tissue necrosis?
liquefactive necrosis
Abscess and pus definitions
accumulation of pus (liquified necrotic tissue), in enclosed space
In brain, what is the cause of liquefactive necrosis?
from ischemia (NOT infection)
what does the brain have a ton of compared to other organs such as kidney or heart?
lipids
End result of liqueatative necrosis (unique too)
dead tissue is removed → cavity or cystic space
gangrenous necrosis cause
insufficient blood to limbs (esp toes) → multiple tissue layers die and undergo coagulative necrosis → gangrene
two situations where person may get gangrene
chronic diseases: poor circulation (diabetes)
trauma or physical injury (forstbite)
wet vs dry gangrene
dry: reduced blood flow: coagulative necrosis
wet: reduce blood flow plus bacterial infection: coagulative → liquefactive necrosis
caseous necrosis: a main cause?
tuberculosis: necrotic core of granuloma → necrotic cells not completely digested (caseous “cheese like”)
caseous necorsis is due to?
body trying to wall off and kill bug with macrophages
focal areas of fat destruction
fat necrosis
fat necrosis causes (2)
trauma to fatty tissue
damage to fat from pancreatitis (inflammation of pancreas: more common)
how does pancreatitis cause fat necrosis: what layer? What cells?
triglycerides from fat cells in mesentery around pancreas → lipase leaks out from damaged pancreatic acinar cells → fatty acids + calcium = saponification
where is there another form of cell death besides necrosis
want to eliminate without inflammation
apoptosis occurs during __ conditions
physiological and pathological
fetal development: embryogenesis: apopotosis: pathological or physioogical?
physiological: get ride of webbing (loss of growth factors)
another purpose of apoptosis
eliminate irreparably damaged cells
Whats similar in mitochondrial and death receptor pathway
Executioner caspase (caspase 3) cleaves proteins → activate enzymes that degrade DNA and proteins, including nuclear matrix and cytoskeleton
morphological appearance of apoptosis
cells shrunken: potassium channels open
nuclei dark and fragmented
growth factor withdrawl, DNA damage, proteins misfolding: what pathway?
mitochondrial
activate death receptors (fas or TNF receptor) pathway
death receptor pathway
death receptor pathways receptors (2)
Fas or TNF
When does blebbing occur? what is the difference?
both in apoptosis and reversible, but they pinch off in apoptosis
apoptosis: shrunken cell, chromatin, condensation
pyknosis
apoptosis: DNA fragmentation
karyorrhexis
what happens to apoptotic bodies? What is the signal for phagocytosis? why?
phosphatidylserine (PS) flips from inner to outer leaflet of plasma membrane, this does NOT cause inflammation
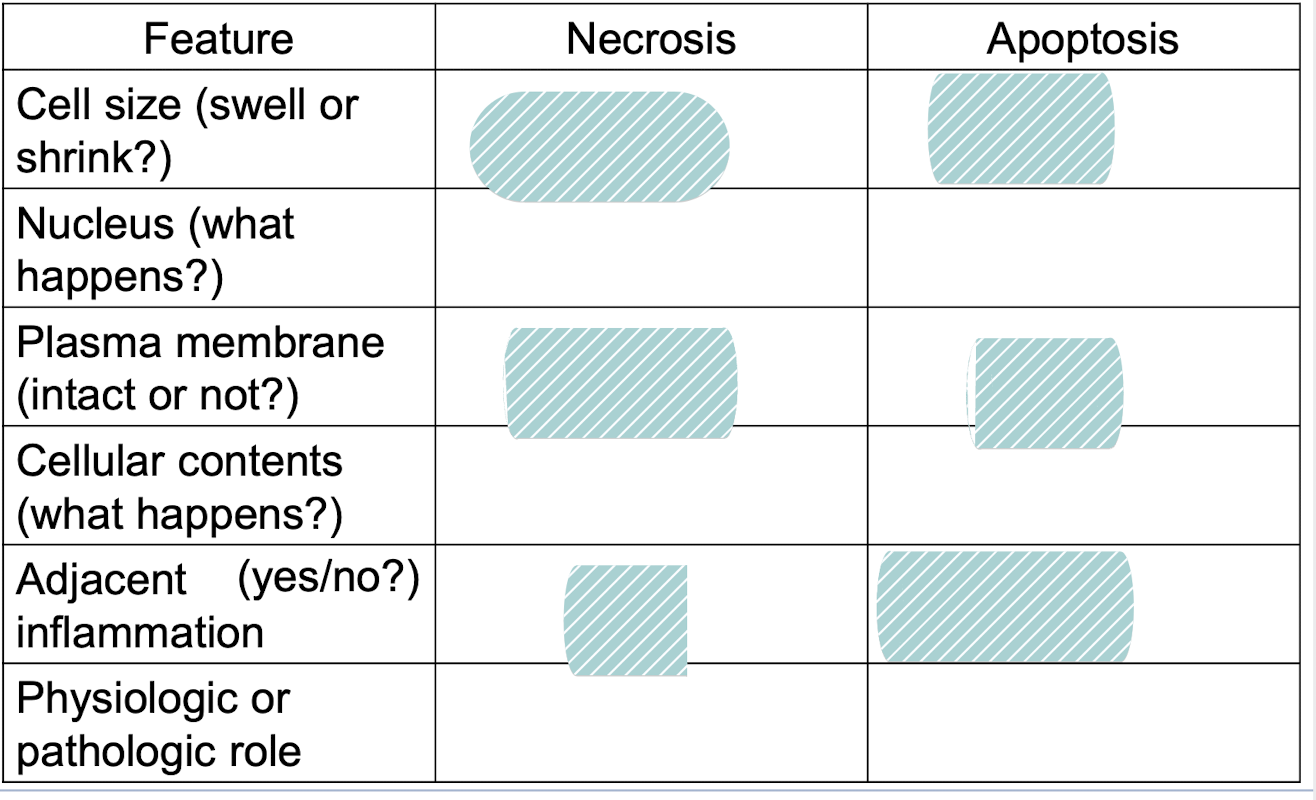
fill it in
enlarged (swelling), reduced (shrinkage)
Pynkosis → karyorrhexis → karyolysis, fragmentation into nueclosome size fragements
Disrupted, intact: (altered structure, esp orientation of lipids)
Enzymatic digestion (can leak out), intact (may be apoptotic bodies)
Frequent, no
Invariably pathologic (culmination of irreversible cell injury), often physiologic, can be pathological (esp DNA and protein damage)
what 3 things does if injuries are irreversible or reversible depend on?
severity, duration, type/status of cell (type, genetics, nutritional status/previous injury)
sometimes necrosis, sometimes apoptosis, why?
depends on type of injury
hypoxia and ischemia causes what? why?
depletion of ATP, cant aerobic
what is hypoxia/ischemia? What does it do to accomodate? (1 thing to cause 3)
lack of O2 → less ATP from oxidative phosphorylation
Activates hypoxia inducible factor 1
increase glycolysis
increase glucose uptake
increase glycogenolysis
Whats more resistant to ischemia or hypoxic?
Liver and skeletal muscle: have more glycogen
What happens to intracellular pH with hypoxia?
more acidic (lower pH) → can inhibit enzyme activity, clumping of chromatin in nucleusi
is hypoxia/ischemia effects (decrease ATP, use glycogen stores, increase glycolysis and lactic acid production) reversible or irreversible?
usually reversible
How does lack of ATP affect cell: why?
protein synthesis:
Cell size:
ER size:
Ribosomes:
more Na+ (less K+), increased solute concentration
decreases
swells
dilates
detach
ischemia effect on cell? (lack of ATP)
impairs activity of Ca++ pump (main signalign pathway) and intracellular Ca2++ rises
How does incrase in intracellular Ca++ contribute to injury? (4)
uncontrolled activation of many cellular enzymes
phospholipase
Protease (degrade enzymes)
Endonuclease
ATPase (help deplete ATP)
How does Ca affect the cytoskeleton?
damages it, detaches from plasma, surge of cytoplasm into area
What helps cause the plasma/organelle membrane and DNA damage with necrosis?
large increase in cytosolic Ca → Ca induced activation of phospholipases, proteases, endonucleases
what are ROS
type of free radical that contains oxygen: (has single unpaired electron in outer orbit)
IF ROS is out of balance ?
oxidative stress
what 3 things can increase ROS and cause oxidative stress>
infection (inflammatory cells that destroy microbes generate ROS), ionizing radiation and some chemicals, ischemia reperfusion injury
How does ROS cause cell injury (3) and conseuqnces of each
lipid peroxidation → damages membranes (reversible then necrosis)
single stranded DNA breaks (apoptosis)
cross-link or fragment proteins (necrosis, apoptosis)
food oxidants, inlammatory products, cigarette smoke, dental material residues → ? associated with what?
oxidative stress in oral cavity, associated with periodontal disease
When is it irreversible (necrosis)?
mitochondrial damage, plasma and lysosomal membrane damage, fragmentation of DNA and chromatin