Melting point analysis
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Why is Melting point analysis used?
•Easy (cheap) check on purity of a crystalline drug substance
What do impurties do to the melting point ? and why?
•Impurities lower melting point
•How? Only one of these is correct!
oOption 1: weaken crystal lattice and lower melting point
oOption 2: do nothing to crystal but stabilise the (liquid) melt
What is melting point Tm ?
solid and liquid are in equilibrium
How do impurties effect solids and liquids?
Impurities can't affect solid (close-packed) but can mix with liquid
Liquid (melt) stabilised by entropy of mixing
Liquid more stable than solid so equilibrium shifts and melting point drops
How to calculate Tm?
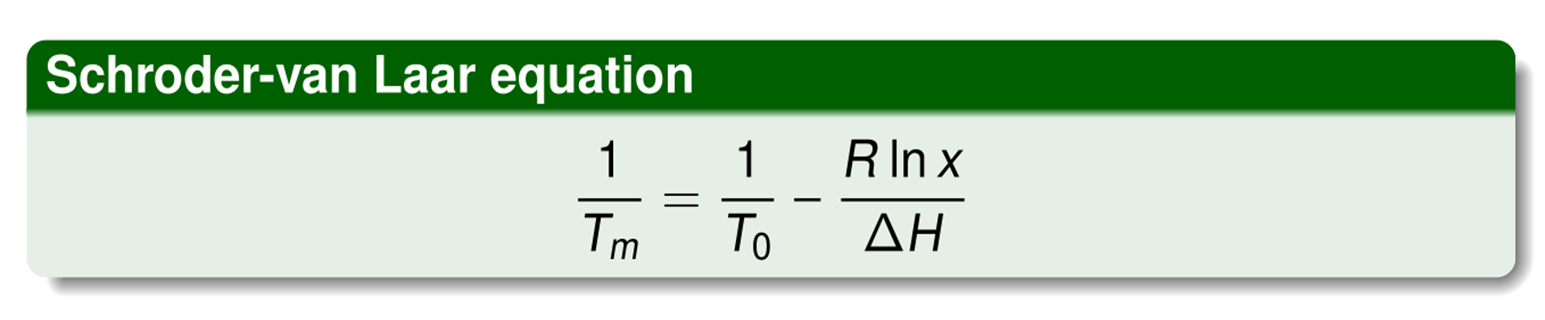
Tm is measured melting point of sample of interest, T0 is melting point of pure substance, ΔH is molar enthalpy of melting, R is ideal gas constant, x is the mole fraction of substance (x=1 is pure material, x=0.9 is 10 % impurity etc)
Summary
•SvL assumes no interactions between different types of molecule, purely entropy of mixing
•Melting point is given on most drug substance bottles
•Can’t use melting point if a drug decomposes near melting point (e.g. aspirin, most proteins, etc)
•If the question is "is my drug substance pure" melting point often the analysis of choice
Questions
•Explain how the SvL equation can be used to determine the purity of a drug substance. How do impurities affect the melting point?
•A drug is reported to have a melting point of 150 °C and a melting enthalpy of 35 kJ/mol. A sample of the drug is found to melt at 145-147 °C. Estimate the percentage by mole purity of the sample. What might the root cause of the lower melting point and how might you confirm this?