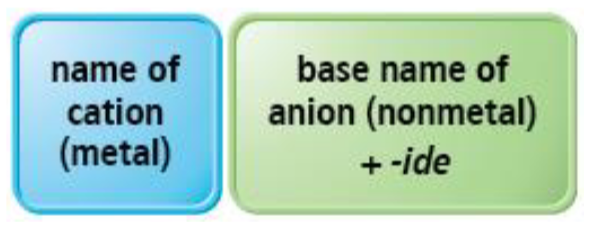
Naming Ionic Compounds
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
71 Terms
Ionic compounds contain a _____ + a non______
metal + nonmetal
How to name ionic compounds with invariant metal
How to name ionic compounds with variant metal

How to deduce formula of a cation and anion
find the lowest whole-number ratio which gives the compound a neutral charge
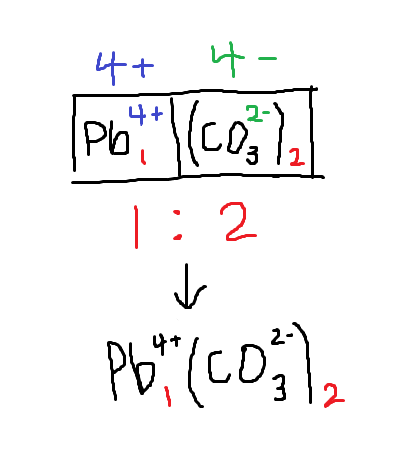
monoatomic vs polyatomic anion
mono = 1 element, poly = multiple elements
most polyatomic anions are ____anions
oxyanions (oxygen + another element)
How to name oxyanions
based on the number of oxygen atoms or the charge of the M/NM on left side of the ion

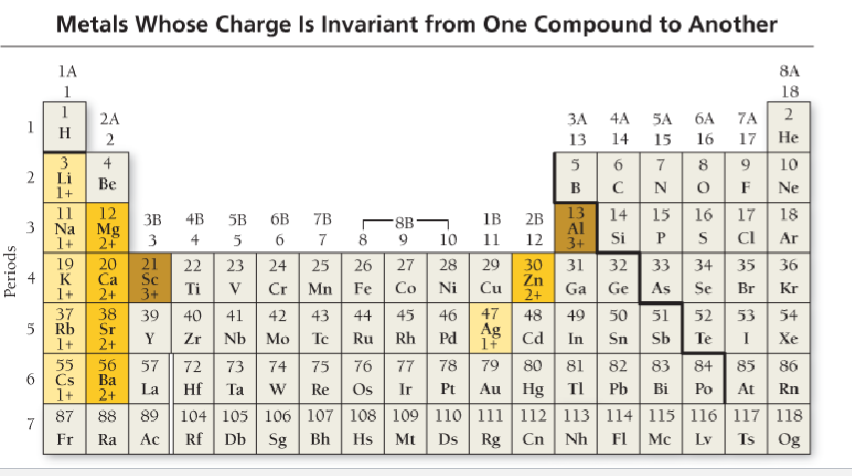
Invariant elements
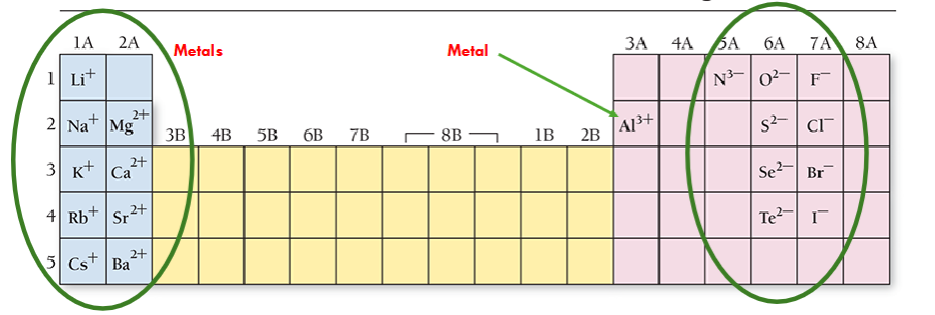
Invariant metals
Monoatomic anions
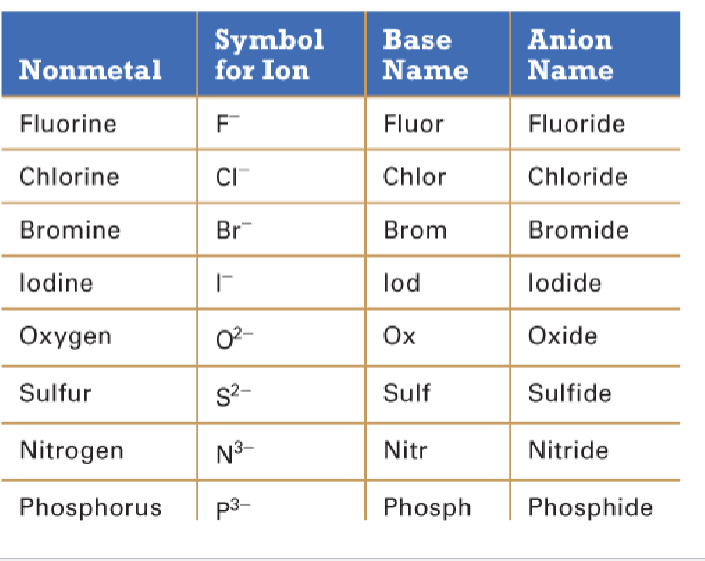
Invariant
One predictable charge
Variant
Charge can vary
Name molecular compounds (NM+NM)
The first element is the more metal-like; smallest group number, then greatest row number.
Common Variant Metal Charges
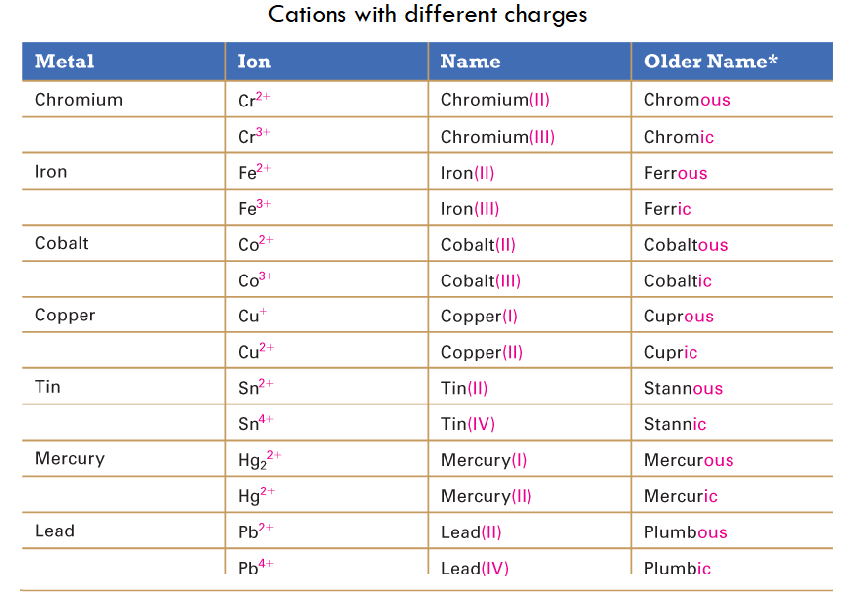
molecular compound prefixes
Mono=1
Di=2
Tri=3
Tetra=4
Penta=5
Hexa=6
Hepta=7
Octa=8
Nona=9
Deca=10
FeCl3
iron(III) chloride
FeCl2
iron(II) chloride
Cr2O3
chromium(III) oxide
Cu3N2
copper(II) nitride
PbCl4
lead(IV) chloride
FeS
iron(II) sulfide
RuO2
Ruthenium(IV) oxide
Ag1+ and N3-
Ag3N
Rb2S
Rubidium sulfide
KF
Potassium Fluoride
MgF2
Magnesium Fluoride
Al2S3
Aluminum Sulfide
ClO-
Hypochlorite
ClO2-
chlorite
ClO3-
chlorate
ClO4-
perchlorate
CO32-
carbonate
Cr Charge
2+, 3+
Fe charge
2+, 3+
Co charge
2+, 3+
Cu charge
1+, 2+
Sn charge
2+, 4+
Hg charge
(Hg2)2+ (I) and Hg2+ (II)
Pb charge
2+, 4+
Li charge
1+
Na charge
1+
K charge
1+
Rb charge
1+
Cs charge
1+
Sc charge
3+
Ag charge
1+
Zn charge
2+
Al charge
3+
N charge
3-
O charge
2-
S charge
2-
Se charge
2-
Te charge
2-
F charge
1-
Cl charge
1-
Br charge
1-
I charge
1-
P charge
3-
Polyatomic anions
NaMnO4
Sodium Permanganate
FeSO4
iron(II) sulfate
Sn(ClO3)2
tin(II) perchlorate
Co3(PO4)2
Cobalt(II) Phosphate
the mono prefix is removed before the 1st NM (molecular compounds)
True / False
True
NO2
nitrogen dioxide
N2O
dinitrogen monoxide
NI3
nitrogen triiodide
PCl5
phosphorus pentachloride
P4S10
tetraphosphorus decasulfide
N2O5
dinitrogen pentaoxide
PBr3
Phosphorus tribromide