CHOLINERGIC DRUGS WANG
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
118 Terms
Autonomic nervous system processes
heart rate
blood pressure
respiration
digestion
urination
sexual arousal
autonomic nervous system. parasympathetic =? sympathetic =?
Autonomic Nervous System Quick Chart
Feature | Parasympathetic (Cholinergic) | Sympathetic (Adrenergic) |
|---|---|---|
Nickname | “Rest & Digest” | “Fight or Flight” |
Preganglionic soma | Brainstem & sacral spinal cord | Thoracic & lumbar spinal cord |
Preganglionic NT | Acetylcholine (ACh) | Acetylcholine (ACh) |
Postganglionic soma | Near target organ | Sympathetic ganglia (near spinal cord) |
Postganglionic NT | Acetylcholine (ACh) or Nitric Oxide | Norepinephrine (NE) (sometimes Epinephrine from adrenal medulla) |
Effects | ↓ Heart rate, ↑ Digestion, Pupil constriction | ↑ Heart rate, ↓ Digestion, Pupil dilation |
Memory Hack | ACh → ACh = “Calm-Calm” | ACh → NE = “Alert-Adrenaline” |
Parasympathetic Nervous System (“Rest & Digest”)
Main Role: Relax or reduce body’s activities.
👁 Eyes: Pupils constrict (better close-up vision, less light enters).
👄 Nose & Mouth: Increases saliva & mucus (digestion, breathing at rest).
🫁 Lungs: Constricts airways (less airflow needed at rest).
❤ Heart: Slows heart rate & reduces pumping force.
🍽 Digestive Tract: Boosts digestion, pancreas releases insulin, stores nutrients.
🚽 Waste Removal: Relaxes bladder & intestines (urination, defecation).
Clinical notes:
Myasthenia gravis (treated with AChE inhibitors)
Dementia (AChE inhibitors improve cognition)
Cold meds may block parasympathetic (dry mouth, urinary retention)
Sympathetic Nervous System (“Fight or Flight”)
Main Role: Activate body for stress/emergency.
👁 Eyes: Pupils dilate (let in more light, improve vision).
❤ Heart: Increases heart rate (more oxygen delivery).
🫁 Lungs: Relaxes airways (improves oxygen intake).
🍽 Digestive Tract: Slows digestion (energy goes to muscles).
🏋 Liver: Releases glucose (quick energy).
🧪 Adrenal glands: Release adrenaline (epinephrine).
🚽 Bladder: Relaxes (delays urination).
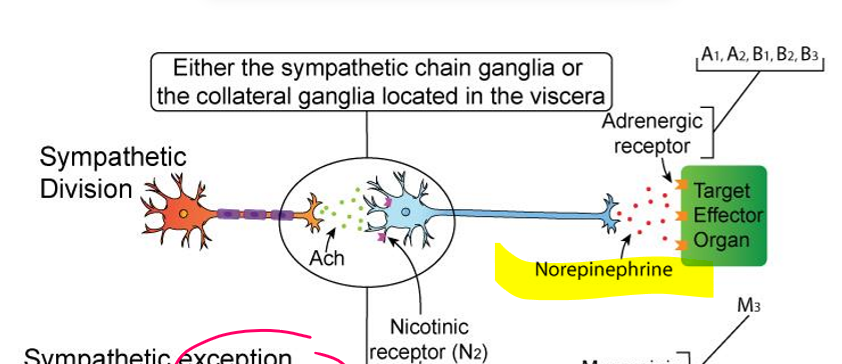
sympathetic nervous system division (typical)
Preganglionic neuron:
Neurotransmitter = ACh
Receptor = Nicotinic (Nn) on sympathetic ganglion
Postganglionic neuron:
Neurotransmitter = Norepinephrine (NE)
Receptor = Adrenergic (α1, α2, β1, β2, β3) on target organ
➡ ACh → NE
sympathetic sweat glands (EXCEPTION)
Preganglionic neuron: ACh → Nicotinic
Postganglionic neuron: ACh → Muscarinic (M3)
➡ ACh → ACh
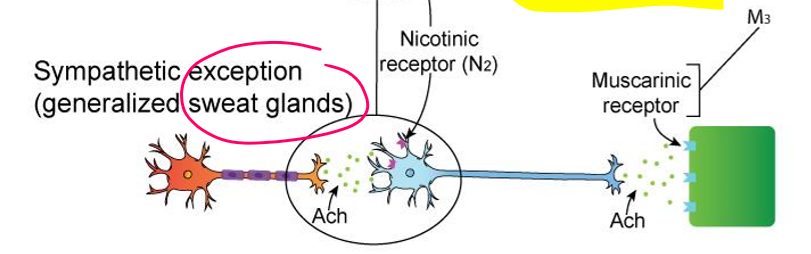
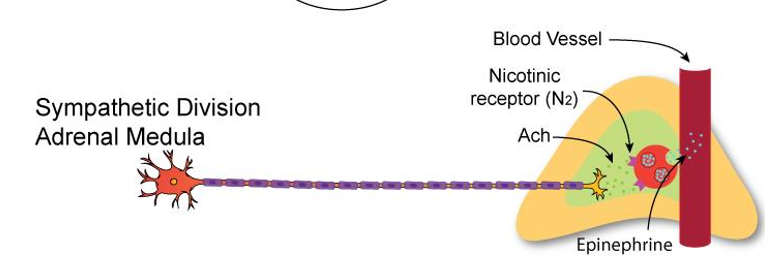
Sympathetic – Adrenal Medulla (Modified Ganglion, Not Exception)
Preganglionic neuron:
ACh → Nicotinic receptors on chromaffin cells
Chromaffin cells (modified postganglionic):
Release Epinephrine (80%) & Norepinephrine (20%) into bloodstream
Hormones act on adrenergic receptors (α, β) throughout body
➡ ACh → Epi/NE (hormonal release)
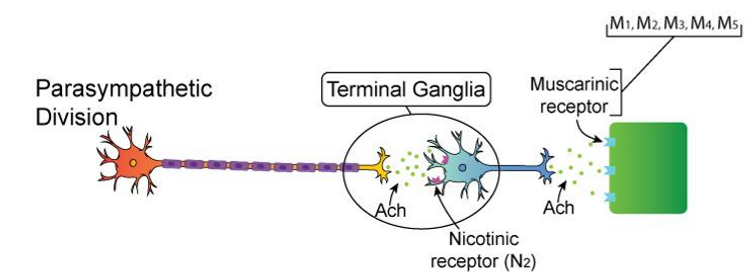
Parasympathetic (Rest & Digest)
Preganglionic neuron:
Neurotransmitter = ACh
Receptor = Nicotinic (Nn) on ganglion
Postganglionic neuron:
Neurotransmitter = ACh
Receptor = Muscarinic (M1–M5) on target organ
➡ ACh → ACh
autonomic nervous system receptors (MUST KNOW)
M2: open K channels, inhibits adenylyl cyclase
in myocardium, smooth muscle, presynaptic sites, CNS neurons
M4: similar to M2
CNS neurons, vagal nerve endings
Alpha2: inhibits adenylyl cyclase, decrease cAMP
presynaptic adrenergic nerve terminals, platelets, lipocytes, smooth muscle
Acetylcholine & Cholinergic Receptors
Acetylcholine (ACh):
Released by cholinergic neurons in ANS, somatic NS, & some CNS neurons.
Endogenous ligand (made by body).
Exogenous mimics: muscarine & nicotine.
Receptor types:
Muscarinic (mAChR) → GPCRs (M1–M5)
Nicotinic (nAChR) → Ligand-gated ion channels
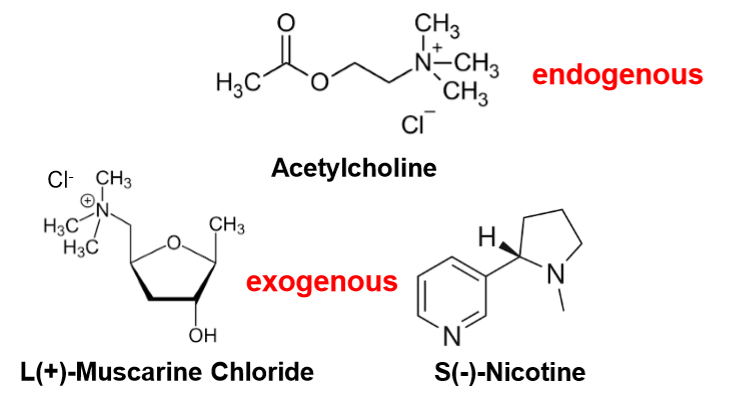
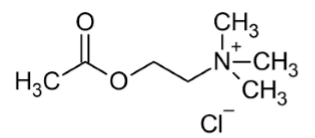
acetylcholine
endogenous
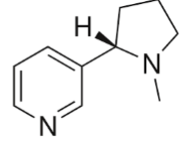
S(-)-Nicotine
exogenous\
Feature | Nicotinic (nAChR) |
|---|---|
Type | Ligand-gated ion channel |
Speed | Fast, excitatory |
Mechanism | ACh → channel opens → ion flow |
Key Pathways | Na⁺ & Ca²⁺ influx, K⁺ efflux → depolarization |
Examples | Skeletal muscle contraction, ganglionic transmission |
Memory Tip | “Door” (direct ion flow) |
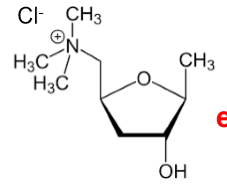
L(+)-Muscarine Chloride
exogenous
Feature | Muscarinic (mAChR) |
|---|---|
Type | GPCR (M1–M5) |
Speed | Slow, modulatory |
Mechanism | ACh → G-protein → 2nd messengers |
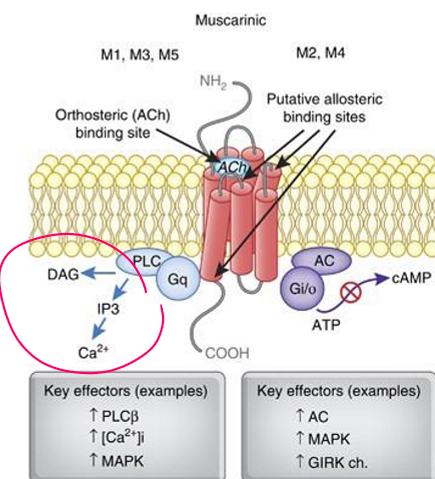
Key Pathways | - Gq (M1, M3, M5): PLC → IP₃, DAG → ↑ Ca²⁺ - Gi (M2, M4): ↓ cAMP |
Examples | Smooth muscle contraction, gland secretion, ↓ HR |
Memory Tip | “Switchboard” (indirect signaling) |
Muscarinic Receptors (mAChR) AFFINITY RANKING
M>A>N
High affinity: Muscarine (fits best)
Medium affinity: Acetylcholine (ACh) (endogenous ligand)
Low affinity: Nicotine
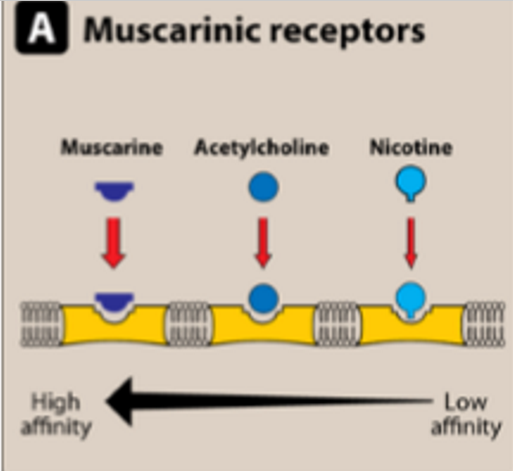
Nicotinic Receptors (nAChR)
N>A>M
High affinity: Nicotine (fits best)
Medium affinity: Acetylcholine (ACh) (endogenous ligand)
Low affinity: Muscarine
Muscarinic Acetylcholine Receptor Structure
7 transmembrane domains
3 intracellular loops, 3 extracellular loops
GPCR Signaling Basics
Resting state: GPCR bound to GDP on G-protein (inactive).
Activation: Agonist binds → GDP → GTP exchange → G-protein activation.
Signal: Gα + Gβγ dissociate → activate effectors (adenylyl cyclase, PLC, ion channels).
Reset: GTP hydrolysis → back to GDP-bound state.
Full Agonist
Maximally activates receptor.
Shifts equilibrium to active state.
Produces maximum biological response.
Partial Agonist
Activates receptor but less than full agonist.
Produces submaximal response, even at high doses.
neutral antagonist
No activity alone, but blocks agonist/inverse agonist.
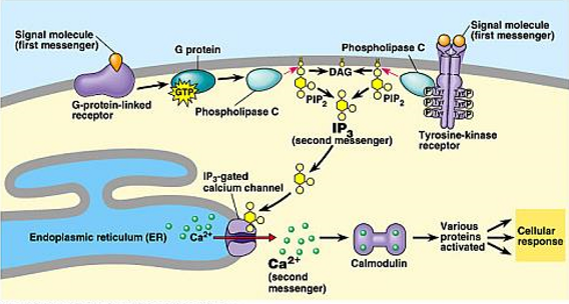
second messengers
molecules that relay signals received at receptors on cell surface
3 major classes of second messengers
cyclic nucleotides
Lipid-Derived Messengers
Calcium Ions (Ca²⁺)
Cyclic Nucleotides
Examples: cAMP, cGMP
Function: Activate Protein Kinase A (PKA) and Protein Kinase G (PKG)
Lipid-Derived Messengers
Examples: Inositol trisphosphate (IP₃), Diacylglycerol (DAG)
Source: Produced from PIP₂ by phospholipase C
Functions:
IP₃: Opens ligand-gated Ca²⁺ channels in ER → ↑ Ca²⁺ release
DAG: Activates Protein Kinase C (PKC)
Calcium Ions (Ca²⁺)
Concentration gradient: Cytosol ~0.1 μM vs. Extracellular ~1 mM
Function: Bind calmodulin → activate Ca²⁺/calmodulin-dependent kinases
GPCR via Phospholipase C (α1 Adrenergic, Gq)
Receptor type: α1 adrenergic
G protein: Gq using GTP
Pathway:
Signal → activates Gq protein
Gq activates Phospholipase C (PLC)
PLC cleaves PIP₂ → IP₃ + DAG
IP₃ → releases Ca²⁺ from ER
DAG + Ca²⁺ → activate PKC
Outcome: Smooth muscle contraction, secretion, cellular activation.
GPCR via cAMP (β Adrenergic, Gs)
Receptor type: β adrenergic
G protein: Gs
Pathway:
Signal → activates Gs protein
Gs activates Adenylyl Cyclase
Adenylyl cyclase converts ATP → cAMP
cAMP activates PKA
PKA → opens ion channels, modulates Ca²⁺ handling
Outcome: Increased heart rate, relaxation of smooth muscle, energy mobilization.
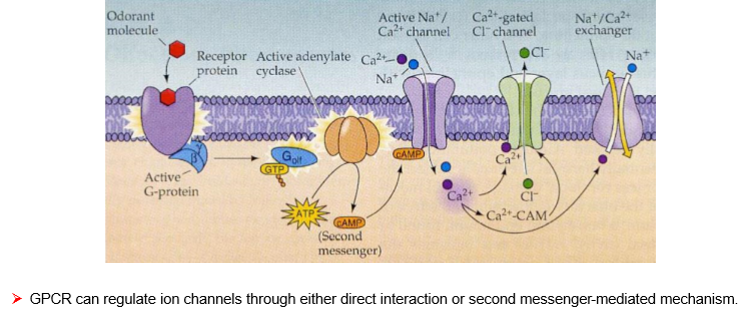
Odd-Numbered mAChRs (M1, M3, M5)
G-protein: Gq/11
Pathway: Activates PLC → IP₃ + DAG → ↑ Ca²⁺ → PKC activation
Effect: Excitatory (depolarization, secretion, contraction)
Locations & Functions:
M1: CNS, gastric/salivary glands → ↑ cognitive function, gastric acid, secretions
M3: Smooth muscle, glands → contraction, ↑ secretion, bladder contraction
M5: CNS, vascular endothelium → dopamine release, vasodilation
➡ Rule: Odd = Excite via Ca²⁺
Even-Numbered mAChRs (M2, M4)
G-protein: Gi/o
Pathway: ↓ Adenylyl cyclase → ↓ cAMP, inhibit Ca²⁺ channels, activate K⁺ channels
Effect: Inhibitory (hyperpolarization, reduced excitability)
Locations & Functions:
M2: Heart, smooth muscle → ↓ heart rate, ↓ contractility
M4: CNS (striatum, midbrain) → inhibition of neurotransmitter release, analgesia, dopamine regulation
➡ Rule: Even = Inhibit via ↓ cAMP
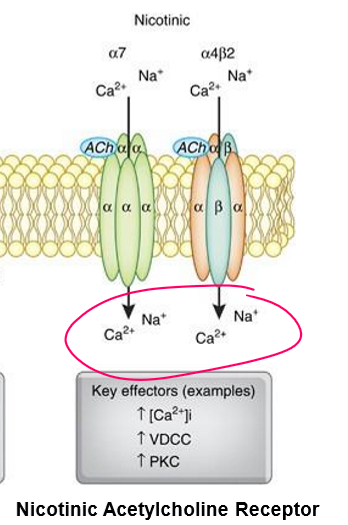
Structure of nAChR
Binding sites: 2 for ACh (on α subunits at αγ and αδ interfaces).
Positive cooperativity: Binding at one site makes binding at the other site easier.
Subunit composition: 5 total subunits (α, β, γ, δ, ε) each with 4 transmembrane domains (M1–M4).
Function of nAChR
Ligand binding → conformational change → opens ion channel.
Ions passed: Na⁺ IN, K⁺ OUT → depolarization of plasma membrane.
Result: Rapid excitatory postsynaptic potential (fast signaling).
parasympathomimetic drug
also called cholinomimetic drug or cholinergic receptor stimulating agent
substance that stimulates parasympathetic nervous system
for glaucoma & underactive bladder
Cholinomimetic (parasympathomimetic): Direct-Acting Cholinergic Drugs
Definition: Stimulate cholinergic receptors directly (mimic ACh).
Types:
Muscarinic agonists (e.g., muscarine, bethanechol, pilocarpine)
Nicotinic agonists (e.g., nicotine, varenicline)
Muscarinic Subgroups: choline esters, alkaloids, others
Uses: Glaucoma (↑ aqueous outflow), urinary retention (stimulates bladder).
Indirect-Acting Cholinergic Drugs
Definition: Increase ACh by inhibiting acetylcholinesterase (AChE).
Classes:
Reversible inhibitors (carbamates): e.g., physostigmine, neostigmine.
Irreversible inhibitors (organophosphates): e.g., echothiophate, pesticides, nerve gases.
Effect: ↑ ACh → prolonged cholinergic signaling.
Uses: Myasthenia gravis, dementia (Alzheimer’s), underactive bladder.
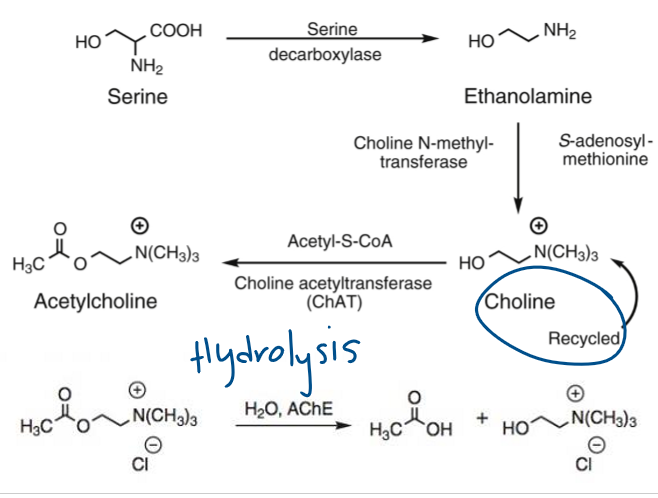
What enzyme synthesizes acetylcholine?
Choline acetyltransferase (ChAT) converts choline + Acetyl-CoA → ACh.
What triggers ACh release into the synaptic cleft?
Calcium influx following depolarization. (Blocked by botulinum toxin.)
How is acetylcholine stored?
In vesicles inside the presynaptic terminal, protected from degradation.
What happens after ACh release?
ACh binds postsynaptic receptors (nicotinic or muscarinic) → intracellular response.
How is ACh degraded?
By acetylcholinesterase (AChE) into choline + acetate.
What happens to choline after ACh breakdown?
Recycled back into the presynaptic neuron for new ACh synthesis.
Easy Notes on Acetylcholine (ACh) Biosynthesis & Hydrolysis
Synthesis
Choline + Acetyl-CoA → Acetylcholine (ACh)
Enzyme: Choline acetyltransferase (ChAT)
Rate-limiting: Choline transport into neuron (blocked by hemicholinium).
Storage
ACh packed into vesicles.
Protected from degradation by vesicular storage.
Release
Ca²⁺ influx (via presynaptic depolarization) triggers vesicle fusion.
ACh released into synaptic cleft.
Blocked by botulinum toxin.
Binding
ACh binds postsynaptic receptors (muscarinic or nicotinic).
Triggers intracellular response.
Degradation
ACh rapidly broken down in cleft by acetylcholinesterase (AChE).
Products: Choline + Acetate.
Recycling
Choline reuptake into presynaptic neuron for reuse.
Key step for maintaining supply.
What bacterium produces botulinum toxin?
Clostridium botulinum
What does botulinum toxin inhibit?
Acetylcholine release from presynaptic neurons.
How does BoNT block ACh release?
Cleaves SNARE proteins → vesicles can’t fuse with the membrane.
What is the result of ACh release inhibition?
Flaccid paralysis (muscle can’t contract).
What condition can botulinum toxin cause if ingested?
Botulism (food poisoning)
What are some medical/cosmetic uses of botulinum toxin?
Botox for wrinkles, treatment of spasticity, migraines, and hyperhidrosis.
What is α-latrotoxin and where does it come from?
A presynaptic neurotoxin from the black widow spider (Latrodectus tredecimguttatus).
What neurotransmitter does α-latrotoxin affect?
Acetylcholine (ACh).
How does α-latrotoxin increase ACh release?
By depolarizing neurons, increasing Ca²⁺ influx, and stimulating uncontrolled exocytosis, TOO ACH released!
What is the main effect of α-latrotoxin on the body?
Excessive ACh release → painful muscle spasms, cramps, possible paralysis.
How does α-latrotoxin differ from botulinum toxin?
α-latrotoxin = promotes excessive ACh release.
Botulinum toxin = blocks ACh release.
What venom is α-bungarotoxin found in?
Cobra venom.
What receptor does α-bungarotoxin target?
Nicotinic acetylcholine receptors (α7 subtype).
How does α-bungarotoxin inhibit ACh signaling?
Binds irreversibly to the receptor → prevents ACh binding → blocks neuromuscular transmission.
What are the effects of α-bungarotoxin?
Muscular weakness, paralysis, and possible respiratory failure.
How is α-bungarotoxin different from botulinum toxin?
Botulinum = blocks ACh release.
Bungarotoxin = blocks ACh receptor binding.
What are the two main types of acetylcholine receptors?
Muscarinic (GPCRs) and Nicotinic (ligand-gated ion channels).
Which muscarinic receptor subtypes are coupled to Gq?
M1, M3, M5 → activate PLC → IP₃ + DAG → ↑Ca²⁺, PKC activation
Which muscarinic receptor subtypes are coupled to Gi?
M2, M4 → inhibit adenylyl cyclase → ↓cAMP, ↓PKA.
What is muscarine, and where is it found?
A non-selective muscarinic agonist found in the Amanita muscaria mushroom.
What are the effects of muscarine poisoning?
Parasympathetic overstimulation → salivation, lacrimation, urination, diarrhea, GI upset, emesis, miosis (SLUDGEM).
What is the antidote for muscarine poisoning?
Atropine (muscarinic receptor antagonist).
what muscarine mimic the function of?
acetylcholine in muscarinic part of the cholinergic nervous system (non-selective agonist of muscarinic acetylcholine receptors)
Give a drug example of enantiomers with different activity.
S(+)-Naproxen sodium = analgesic.
R(−)-Naproxen sodium = inactive.
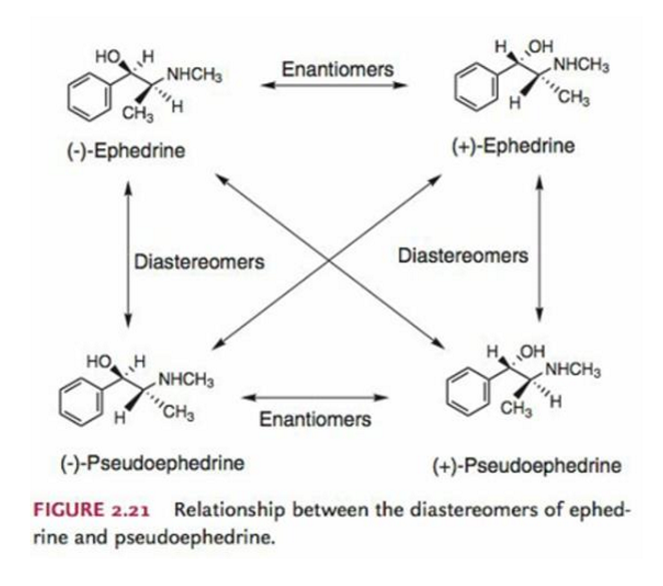
Give a drug example of diastereomers.
Ephedrine vs Pseudoephedrine.
How many stereoisomers are possible for muscarine?
8 (3 chiral centers → 2³).
What is the stereochemistry of L-(+)-Muscarine?
2S,3R,5S.
Why was thalidomide a tragedy?
Given to pregnant women (1957) as a sedative; caused ~10,000 cases of phocomelia (limb malformation) in infants.
What are the enantiomers of thalidomide and their effects?
R-(−)-Thalidomide = sedative.
S-(+)-Thalidomide = teratogenic (birth defects).
Why can’t we just give the safe (R) enantiomer of thalidomide?
Because enantiomers interconvert in the body, making separation ineffective.
giving ONLY the safe one won’t work, the harmful form will still appear
What toxin does Amanita muscaria contain?
Muscarine, a muscarinic receptor agonist.
What symptoms are caused by muscarine poisoning? (mushrooms of the amanita phalloides)
Parasympathetic overstimulation → salivation, lacrimation, urination, diarrhea, bradycardia, miosis, CONVULSIONS, DEATH
What is the antidote for muscarine poisoning?
Atropine (muscarinic antagonist).
What additional treatment is used if CNS excitation/hallucinations occur (because of atropine)?
Benzodiazepines.
Which mushroom species causes fatal liver failure?
Amanita phalloides (death cap) → amatoxins.
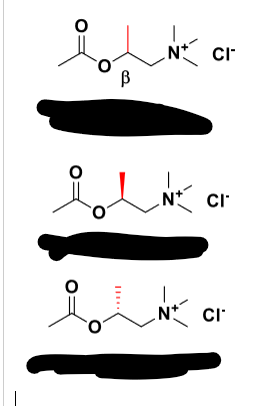
METHACHOLINE
Q: What receptor does methacholine act on?
A: Selective muscarinic agonist (little activity at nAChRs).
Q: Which enantiomer of methacholine is more potent?
A: S-enantiomer (240× more potent at mAChRs).
Q: How resistant is methacholine to AChE hydrolysis?
A: More resistant than ACh (S-enantiomer ~54% slower hydrolysis).
Q: Clinical use of methacholine?
A: Diagnosis of asthma (methacholine challenge test).
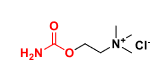
CARBACHOL
Q: What type of drug is carbachol?
A: Carbamate analog of ACh (direct cholinomimetic).
Q: Is carbachol selective?
A: No → agonist at both mAChRs & nAChRs.
Q: Why is carbachol more stable than ACh?
A: Resistant to hydrolysis by acid, base, and AChE.
Q: Clinical uses of carbachol?
A: Glaucoma & inducing miosis in ocular surgery.
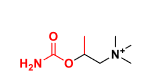
Bethanechol
Q: What receptor does Bethanechol act on?
A: Selective muscarinic agonist (mAChRs only).
Q: Is Bethanechol resistant to AChE?
A: Yes → not hydrolyzed by AChE → long duration of action.
Q: Clinical uses of Bethanechol?
A: Treatment of ileus and urinary retention (often after anesthesia).
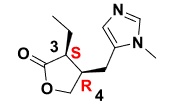
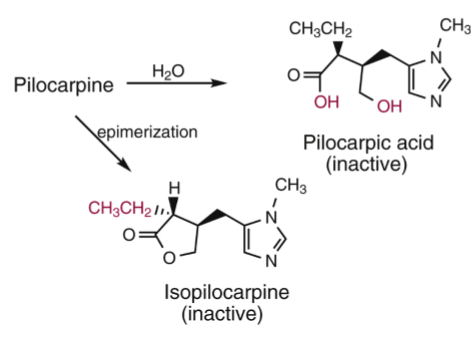
Pilocarpine
Q: What type of drug is Pilocarpine?
A: A natural alkaloid muscarinic agonist.
Q: Which receptor does Pilocarpine primarily act on?
A: M3 muscarinic receptors.
Q: Clinical uses of Pilocarpine?
A:
Sjögren syndrome (stimulates tear & saliva secretion).
Glaucoma (reduces intraocular pressure).
Q: Stability of Pilocarpine?
A: Unstable → prone to hydrolysis and epimerization; needs refrigeration, short shelf-life (~2 weeks).
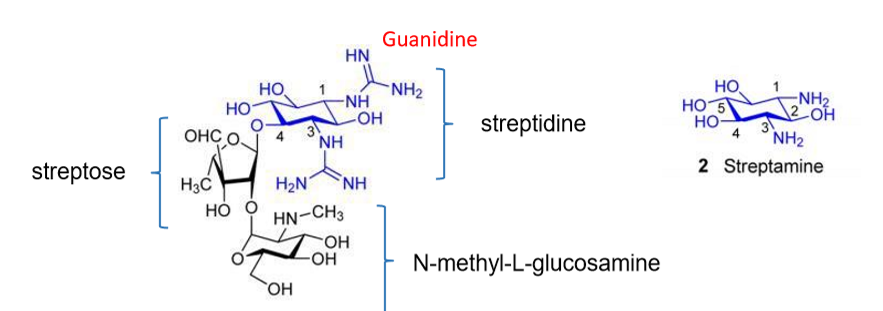
STREPTOMYCIN
Q: Why must streptomycin be given IM or IV?
A: It is polar and highly charged (+3), so it cannot cross membranes.
Q: Can streptomycin cross the blood-brain barrier?
A: No → ineffective for meningitis.
Q: How does streptomycin enter bacterial cells?
A: Binds negatively charged groups on membranes, displaces Mg²⁺/Ca²⁺, creates pores for entry.
Q: What are the main toxicities of streptomycin?
A: Ototoxicity, nephrotoxicity, and drug resistance.
Q: What was streptomycin’s major historical use?
A: First effective drug against tuberculosis.
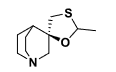
Cevimeline
Q: What type of drug is cevimeline?
A: A muscarinic agonist (quinuclidine analog).
Q: What is cevimeline used for?
A: Dry mouth in Sjögren syndrome (similar to pilocarpine).
Q: What are common side effects of cevimeline?
A: Nausea, vomiting, diarrhea, sweating, rash, headache, runny nose, cough, drowsiness, hot flashes, blurred vision, difficulty sleeping.
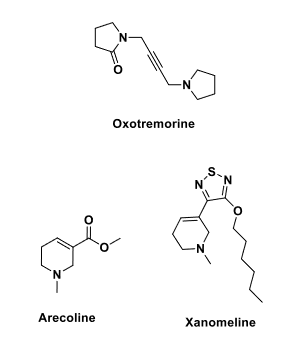
Oxotremorine, Arecoline, Xanomeline
Q: What are oxotremorine, arecoline, and xanomeline used for?
A: Treatment of Alzheimer’s disease.
Q: Why are these drugs used in Alzheimer’s?
A: They are selective muscarinic agonists in the brain → enhance cholinergic transmission.
Q: What neurotransmitter dysfunction is seen in Alzheimer’s disease?
A: Reduction in ACh, serotonin, norepinephrine, dopamine, and glutamate.
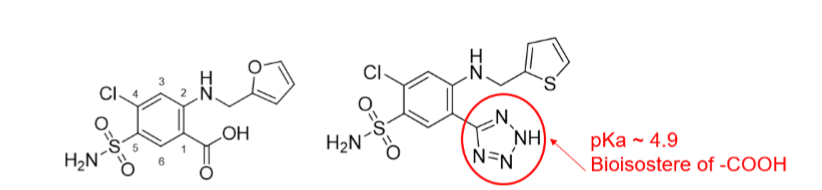
Bioisostere
Q: What is a bioisostere?
A: A functional group that can replace another in a drug while maintaining activity.
Q: Example of a bioisostere replacing –COOH?
A: In furosemide (–COOH) → azosemide (–NH–N=N–NH, pKa ~4.9).
Q: Why do sulfonamides act as PABA analogs?
A: Similar geometry (6.7 Å vs 6.9 Å between amine and acidic groups).
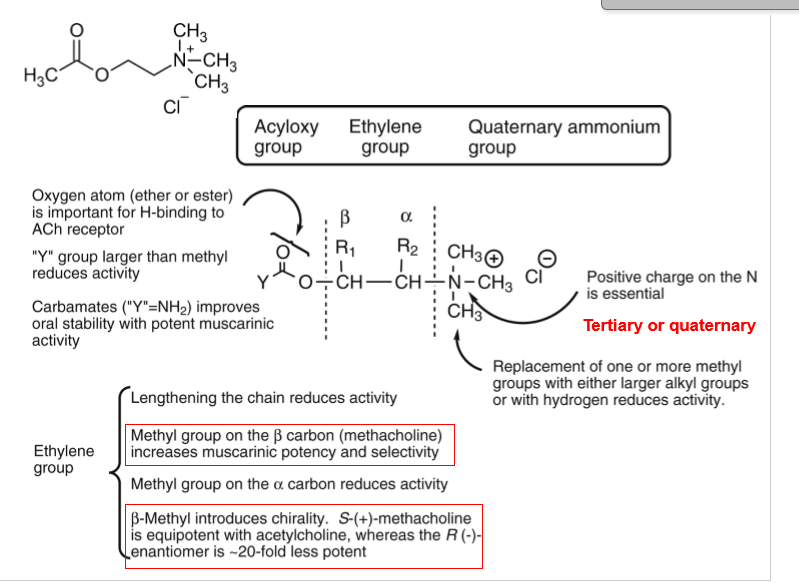
SAR of Muscarinic Agonists
Q: What is Ing’s “Rule of Five”?
A: No more than 5 atoms between cationic nitrogen and terminal H for optimal activity.
Q: How do carbamate analogs of ACh affect stability?
A: Increase stability against hydrolysis → longer duration.
Q: Effect of β-methyl substitution (Methacholine)?
A: ↑ Muscarinic potency and selectivity.
Q: Effect of α-methyl substitution?
A: ↓ Activity.
Q: Which methacholine enantiomer is more potent?
A: S-(+)-Methacholine (20× more potent than R-(–)).
Q: Why is the quaternary ammonium group essential in muscarinic agonists?
A: Provides the positive charge needed for receptor binding.
NICOTINE
Q: What type of drug is nicotine?
A: A nicotinic receptor agonist.
Q: Which receptor subtype mediates nicotine dependence?
A: α4β2 nAChRs → increases dopamine release from neurons.
Q: What are first-line treatments for smoking cessation?
A: Nicotine replacement therapy (NRT), varenicline, and bupropion.
Q: What are some forms of nicotine replacement therapy (NRT)?
A: Oral inhaler, chewing gum, lozenge, nasal spray, extended-release patch.
Q: How does nicotine delivery differ between oral/spray vs patch?
A: Oral/nasal spray = rapid delivery; patch = slower but steady 24h release.
Bupropion
Q: What was bupropion originally approved for?
A: Depression (antidepressant).
Q: How is bupropion used in smoking cessation?
A: As a nAChR antagonist + inhibits dopamine & norepinephrine reuptake.
Q: Brand name of sustained-release bupropion for smoking cessation?
A: Zyban.
Varenicline
Q: What type of drug is varenicline?
A: Partial agonist at α4β2 nAChRs, full agonist at α7 nAChRs.
Q: How does varenicline help with smoking cessation?
A: Produces mild dopamine release (reduces craving) while blocking nicotine binding.
Q: What natural compound is related to varenicline?
A: Cytisine (natural α4β2 nAChR agonist from Laburnum tree).
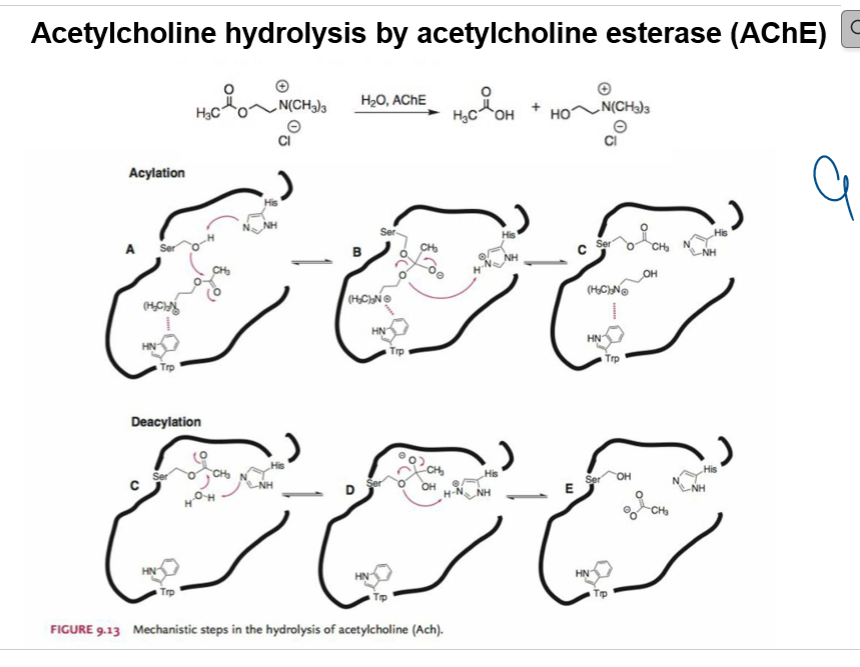
Overall Reaction
Acetylcholine + H₂O —(AChE)→ Acetate + Choline
Steps of Hydrolysis
Binding (Step A)
ACh enters the active site of AChE.
The catalytic triad (Ser, His, Trp) positions ACh.
Acylation (Steps B–C)
Serine’s –OH attacks the carbonyl carbon of ACh’s ester bond.
Tetrahedral intermediate forms.
Choline leaves → acetylated enzyme intermediate.
Deacylation (Steps C–E)
Water enters active site.
H₂O (activated by His) attacks acetyl–Ser bond.
Tetrahedral intermediate collapses → regenerates free Ser–OH.
Acetate released.
Key Concepts
Very fast process → explains why ACh has such a short half-life in the synapse.
Catalytic triad: Ser–His–Trp (sometimes Glu also contributes).
Therapeutic relevance: AChE inhibitors (e.g., physostigmine, organophosphates) prolong ACh activity.
Classification of AChE inhibitors:
Carbamates: neostigmine, pyridostigmine, physostigmine, rivastigmine
Non-carbamates: edrophonium, donepezil, galantamine, tacrine.
Reversible inhibitors
more stable than ACh’s acetylated enzyme, but can regenerate.
Classification of AChE inhibitors
Organophosphates: sarin, malathion, diazinon.
Used as insecticides & chemical warfare agents.
phosphorylate enzyme → very stable, long-lasting, often toxic.
Irreversible inhibitors
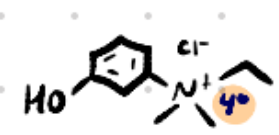
Edrophonium
Structure: Aromatic ring + quaternary ammonium.
Amine type: Quaternary (charged) → does not cross BBB.
Class: Reversible non-carbamate AChEI.
Use: Dx of myasthenia gravis (SHORT-acting “Tensilon test”).
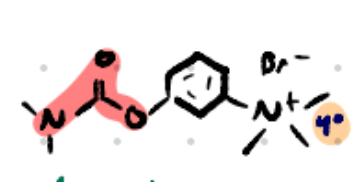
Neostigmine
INDIRECT CHOLINOMIMETIC
NONCOVALENT INHIBITOR
Structure: Aromatic + carbamate + quaternary ammonium.
Amine type: Quaternary (charged) → no BBB crossing.
Class: Reversible carbamate AChEI.
Use: Myasthenia gravis, ileus, reversal of NMJ blockade.
POST OPERATION
P.E.N (pyridostigmine, edrophonium, neostigmine)
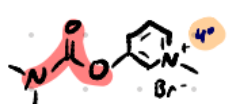
Pyridostigmine
Structure: Pyridine ring + carbamate + quaternary ammonium.
Amine type: Quaternary (charged) → no BBB crossing.
Class: Reversible carbamate AChEI.
Use: Myasthenia gravis (longer acting than neostigmine) prolong ACh action
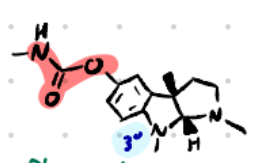
Physostigmine
Structure: Indole nucleus + carbamate.
Amine type: Tertiary amine (uncharged) → crosses BBB.
Class: Reversible carbamate AChEI (prototype).
Mechanism: Forms carbamylated AChE (slower hydrolysis vs acetylated enzyme).
Use: Glaucoma, atropine overdose (ANTIDOTE FOR ATROPINE), anticholinergic toxicity
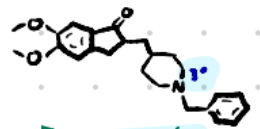
Donepezil
Structure: Large aromatic piperidine system, no carbamate.
Amine type: Tertiary amine → crosses BBB.
Class: Reversible non-carbamate AChEI.
Use: Alzheimer’s disease.
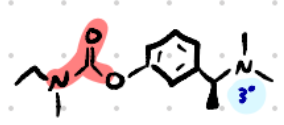
Rivastigmine
Structure: Aromatic + carbamate.
Amine type: Tertiary amine → crosses BBB.
Class: Reversible carbamate AChEI.
Use: Alzheimer’s, Parkinson’s dementia.
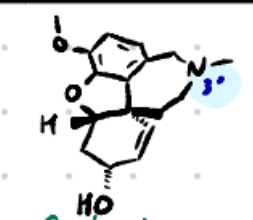
Galantamine
Structure: Polycyclic alkaloid with tertiary nitrogen.
Amine type: Tertiary amine → crosses BBB.
Class: Reversible non-carbamate AChEI.
Use: Alzheimer’s disease.
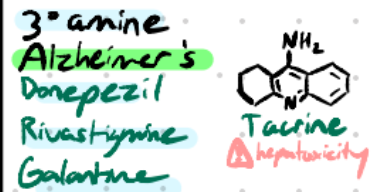
HEPATOXICITY
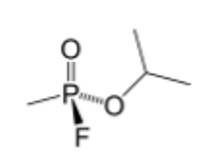
Sarin
Type: G-series nerve agent (organophosphate, phosphonate ester)
Properties: Volatile, hydrolysis-resistant
Mechanism: Irreversible AChE inhibition → ↑ACh → continuous stimulation
Onset: Rapid, short persistence
Notes: Aging makes reversal harder