Structure 1.3 Electron configurations
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Radio waves
Long wavelength
Low frequency
Low energy
Gamma rays
Short wavelength
High frequency
High energy
Visible light: red light
Long wavelength (700nm)
Low frequency
Low energy
Visible light: violet light
Short wavelength (400nm)
High frequency
High energy
Continuous spectrum
Shows all wavelengths of visible light

Absorption line spectrum
Black lines on a colored background (certain wavelengths missing).

Emission line spectrum
Coloured lines on a black background (only certain wavelengths visible)

Hydrogen emission spectrum
656 nm (red line): transition from n=3 to n=2
486 nm (turquoise): transition from n=4 to n=2
434 nm (blue line): transition from n=5 to n=2
410 nm (violet line): transition from n=6 to n=2

Absorbing photons of energy
Electrons are promoted to higher energy levels (excited state)
Emitting photons of energy
Electrons transition to lower energy levels
Electron transitions to n=1
Emit UV radiation (electron transitions emitting the greatest amount of energy)
Electron transitions to n=2
Emit visible light
Electron transitions to n=3
Emit infrared radiation (electron transitions emitting the greatest amount of energy)
Atomic orbitals
A region of space where there is a high probability of finding an electron.
S orbitals
Spherical

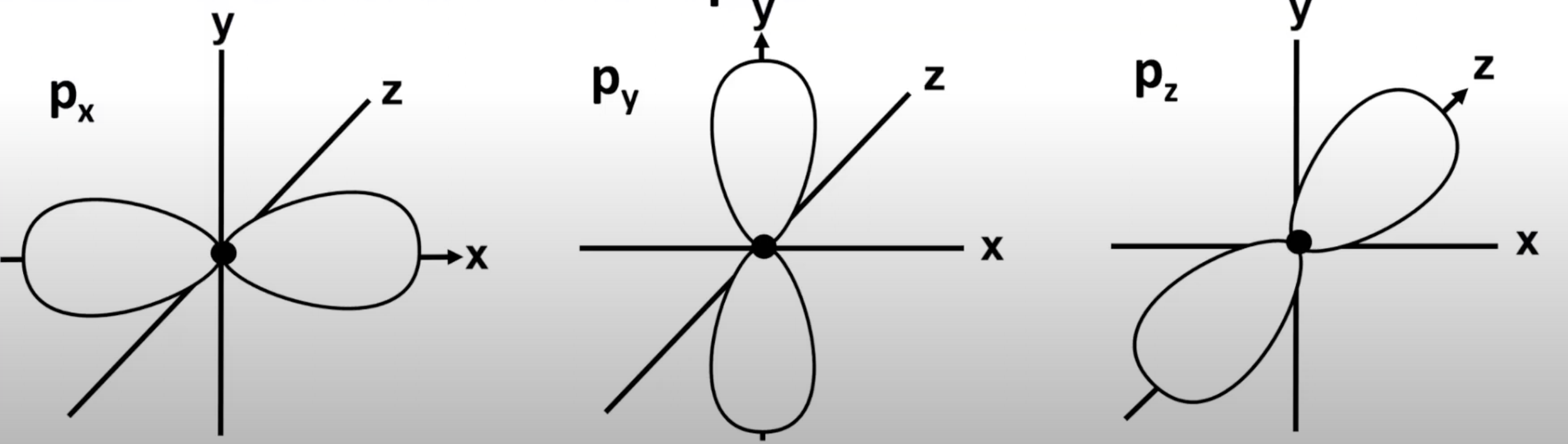
P orbitals
Dumbbell shaped. Px, Py and Pz are at 90 degrees to each other.
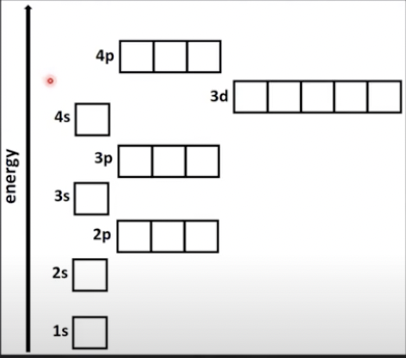
Sublevels organized in order of energy
Within a main energy level the order of energy is:
s < p < d < f
Electron configuration
A representation of the arrangement of electrons within an atom or ion.
E.g helium: 1s²
1 = main energy level
s = sub-level
2 = number of electrons in sub-level
Electron configuration for carbon, C
1s² 2s² 2p² or [He] 2s² 2p²
Electron configuration for sodium, Na
1s² 2s² 2p⁶ 3s¹ or [Ne] 3s¹
Condensed/abbreviated electron configurations
Use the symbol of a noble gas to represent the core electrons
[He] = 1s²
[Ne] = 1s² 2s² 2p⁶
[Ar] = 1s² 2s² 2p⁶ 3s² 3p⁶
The Aufbau principle
Used to determine the electron config. of an atom or ion.
Electrons fill lowest energy sub-levels first, 1s 2s 2p 3s 3p 4s 3d 4p etc.
Pauli exclusion principle
An atomic orbital can hold a maximum of two electrons and they must have opposite spins.
Hund’s rule
Degenerate orbitals (orbitals with the same energy) are filled singly with the same spin before being doubly occupied.
Electron configurations 4s 3d sublevels
K = [Ar] 4s¹
Ca = [Ar] 4s²
Sc= [Ar] 4s² 3d¹
Ti = [Ar] 4s²
V = [Ar] 4s² 3d³
Cr = [Ar] 4s¹ 3d⁵
Mn = [Ar] 4s² 3d⁵
Fe = [Ar] 4s² 3d⁶
Co = [Ar] 4s² 3d⁷
Ni = [Ar] 4s² 3d⁸
Cu = [Ar] 4s¹ 3d¹⁰
Zn = [Ar] 4s² 3d¹⁰
Positive ions (cations)
Are formed when atoms lose electrons.
The electrons are lost from the highest energy level first.
When transition elements form cations, they lose their 4s electrons first.
Negative ions (anions)
Are formed when atoms gain electrons.
Convergence limit
The frequency at which the spectral lines converge.
The energy levels converge when n=∞
The spectral lines for each series (UV, visible light and IR) also converge at higher energy.
Ionisation energy
The energy required for the electron transition from n=1 to n=∞
Can be calculated using the frequency (or wavelength) of the convergence limit.
E = hv
energy (J) = Planck’s constant (6.63 × 10−34 J*s) x frequency (s−1)
c = vλ
speed of light (3.00 × 10⁸ m s−1) = frequency (s−1) x wavelength (m)
Successive ionisation energies
The ionisation energy increases as electrons are removed from an increasingly positive ion → causing an increase in attraction between the nucleus and the remaining electrons.
Big ionisation energy caused by:
Different energy levels
Doubly/singly filled electron orbitals