Measurement of the specific latent heat of fusion of ice
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
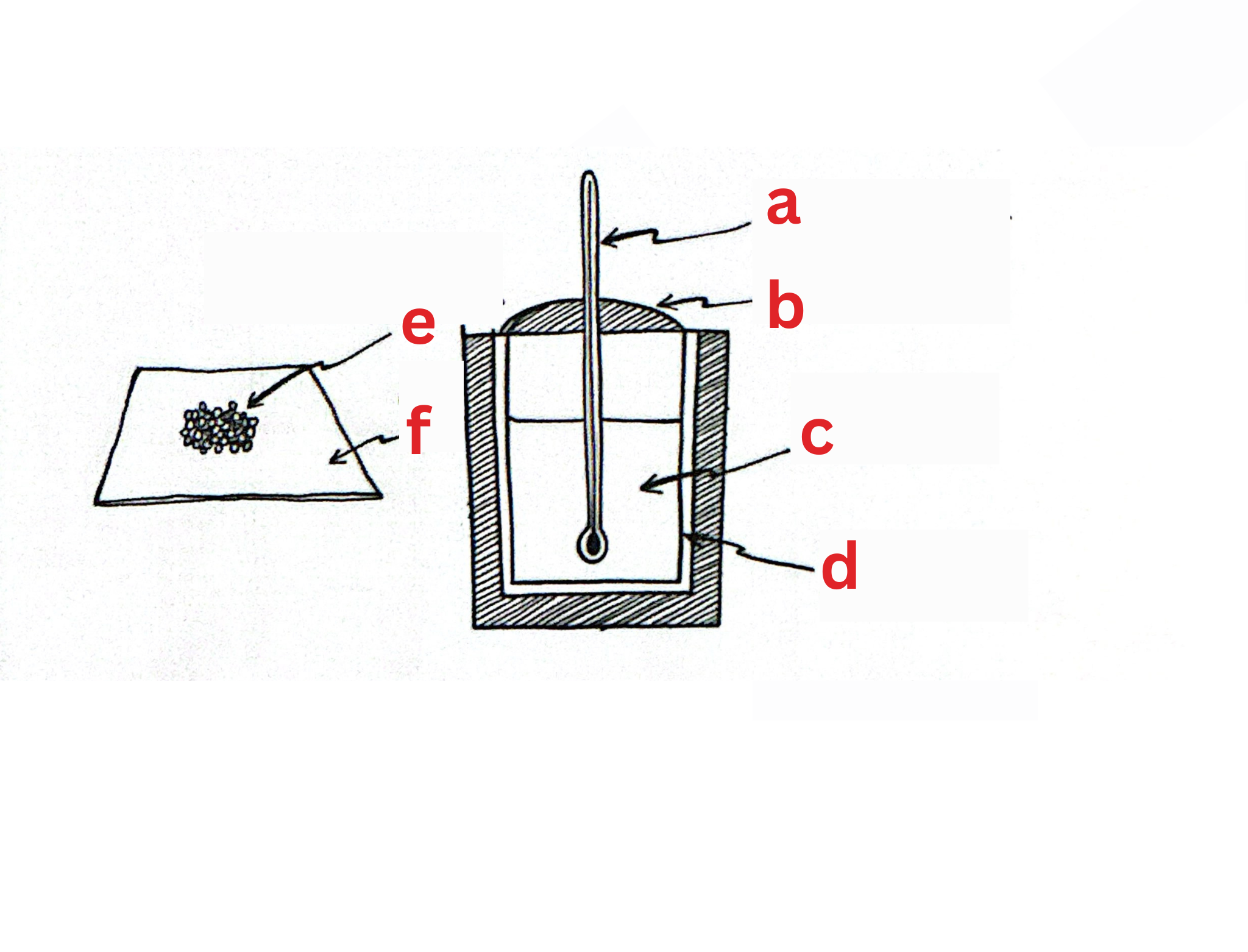
Label This Diagram
a - thermometer
b - lid
c - warm water
d - calorimeter
e - crushed, melting, dried ice
f - cloth
Demonstrate the method of this experiment • 1
Measure mass of calorimeter
Add warm water
Measure mass of calorimeter and water
Calculate mass of water
Demonstrate the method of this experiment • 2
Measure initial temperature
Add crushed and dried ice
Measure final temperature
Calculate temperature change
Demonstrate the method of this experiment • 3
Measure mass of calorimeter and water and ice
Calculate mass of ice
Define 3 precautions that are associated with this experiment • 1
Make sure ice is melting - latent heat at 0°C
Define 3 precautions that are associated with this experiment • 2
Crush and dry ice for no excess water
Define 3 precautions that are associated with this experiment • 3
Room temperature between initial water temperature and 0°C to cancel out errors
Write out the formula associated with this experiment
m (i) l (fi) + m(i) c(w) temp change (i) = m (w) c (w) temp change (w+c) + m (c) c (c) temp change (w + c)