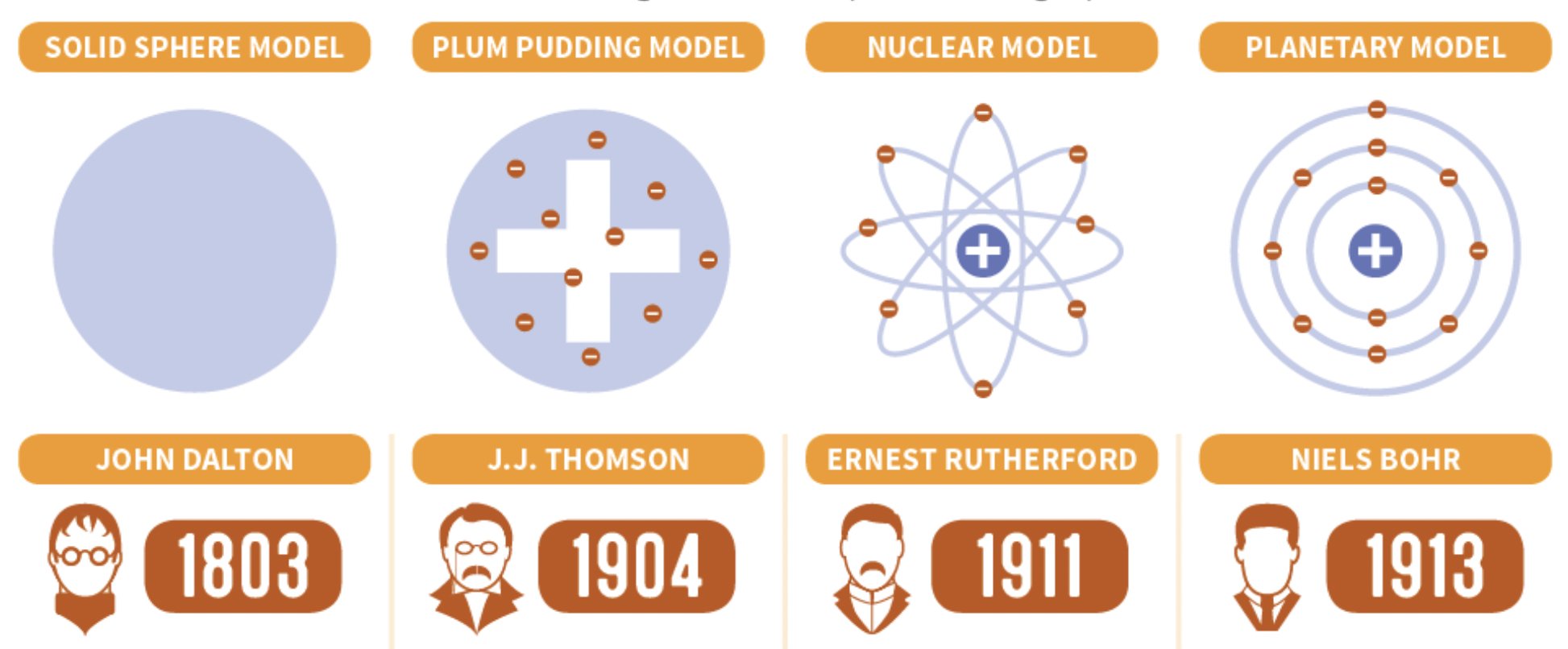
Atomic Theory and Related Famous Experiments
(Scroll to bottom for simplified chart)
In order from earliest to latest:
Democritus
All matter is made up of atoms, which are very small, indestructible solid particles that are separated by empty space
- INACCURACY: though he says that atoms are too small to be seen individually (true if referring to with human sight), they can be seen under magnification
Proposed atoms were in constant random motion, when enough bumped into each other, they would stick to form clumps that grow into visible matter
Democritus was a philosopher, not a scientist; his idea of the atom was only a guess, but very accurate
Dalton
All substances are made of atoms, the smallest particles of matter. They cannot be divided, created, or destroyed.
- INACCURACY: atoms are not the smallest particles of matter--subatomic particles like electrons are even smaller.
All atoms of the same element are alike and have the same mass. Atoms of different elements are different and have different masses.
Atoms join to form compounds, which always consist of the same atoms in the same proportions.
“Billiard Ball” Model
J.J. Thomson
Discovered electrons
- Electrons - negatively charged subatomic particles
Cathode ray experiment
Theorized that the atom was mostly positively charged matter (plum pudding) with negatively charged electrons scattered throughout
“Plum Pudding” Model
Rutherford
Discovered the nucleus
Gold foil experiment
- Shot a beam of alpha particles (alpha particles are positively charged) at a very thin sheet of gold foil, with a circular screen surrounding it that glowed when hit by alpha particles
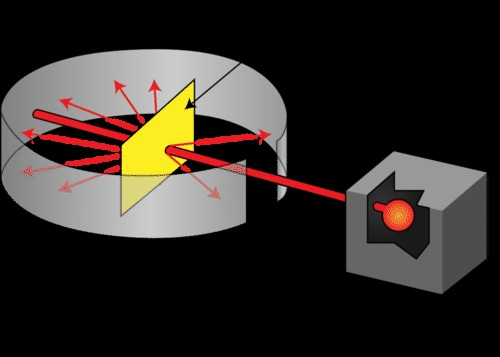
- Most of the particles passed through the foil, while some bounced off or deflected their path
Rutherford’s Atomic Theory
There is one small area in the atom called the nucleus, containing all of the positive charge and virtually all of the mass of an atom
Rest of atom is mostly empty space + electrons
Discovered positively charged particles in the nucleus, which he named protons
Predicted neutrons, but failed to find them
- Student James Chadwick discovered them years later
Rutherford Model
Bohr
Electrons can move about the nucleus only at fixed distances based on their energy
Called the fixed distances energy levels, or electron shells
- Nucleus at centermost position, electron shells encase it
Electrons with less energy are at lower energy levels, closer to the nucleus
Electrons with more energy are at higher energy levels, farther from the nucleus
If an electron absorbs the right amount of energy, it would jump to the next higher energy level, and could also move to a lower energy level if it lost that same amount of energy
An electron cannot exist in between two energy levels
- Ladder metaphor - you can only stand on the rungs of the ladder but not the spaces between
Bohr model