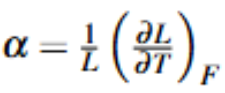Properties of Matter Equations
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Thermal Energy of a system
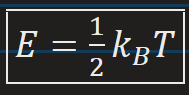
The Ideal Gas Equation
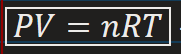
Volume thermal expansivity
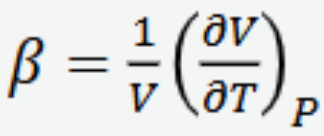
Young’s modulus
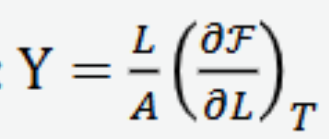
Isothermal bulk modulus
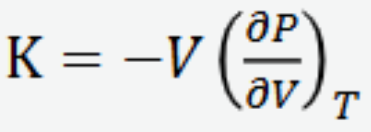
Isothermal compressibility
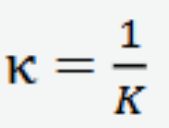
The equations of 3D work on reversible processes
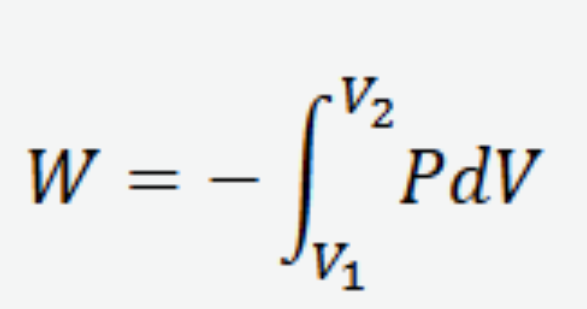
Adiabatic process change in internal energy
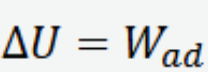
First Law of thermodynamics
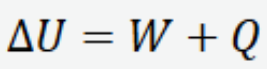
Planck’s Distribution Law
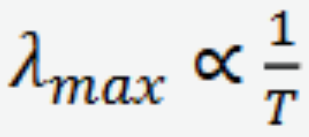
Heat capacity
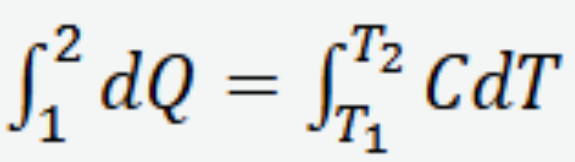
Plane spacing for cubic crystals
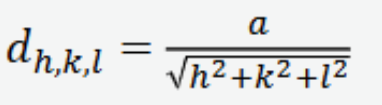
Bragg equation for X-ray diffraction
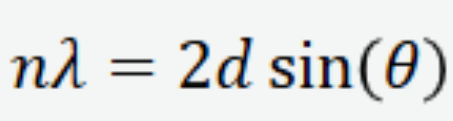
average kinetic energy of a large number of molecules
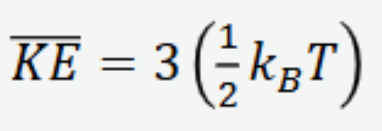
average velocity of gas molecules
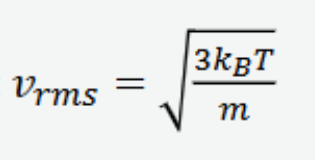
Change in internal energy relation to temperature for an ideal gas
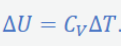
Difference in isobaric C - Isochoric C
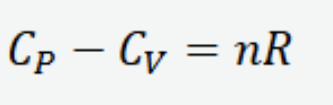
Temperature and volume relation for an adiabatic process of an ideal gas
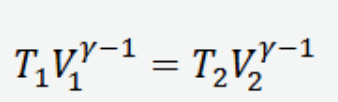
Pressure and Volume relation for an adiabatic process on an ideal gas
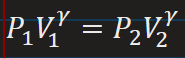
adiabatic exponent (gamma)
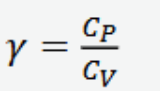
Internal energy of n moles of atoms in a crystal when all 6 dof are accessible
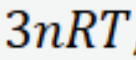
Dulong-Petit law at ‘higher temperatures’
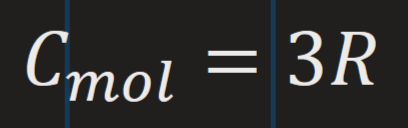
Debye temperature
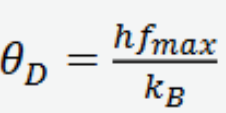
Change in entropy
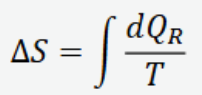
Mean free path
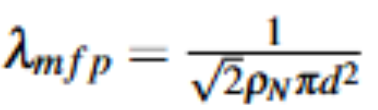
Linear Expansivity