Stoichiometry and General Concepts
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
moles
SI unit for the amount of any substance
Avogadro’s number: 6.022 × 1023 molecules
molar mass
sum of the mass of all atoms found in one mole’s worth of the substance
Units: grams per mole
Multiply the atomic mass by its coefficients and add them together
how do you calculate number of moles in a sample?
n= m/M
m= mass in grams of sample (g)
M= molar mass (g/mol)
mole ratio
ratio between the amount of moles of any two molecules involved in a chemical reaction
comparison between the coefficients of any two molecules in a chemical reaction
percent composition
the percent of the compound’s mass that is made up by that element

molecular formula
tells you the exact number of atoms of each element in a compound
empirical formula
simplest/most reduced ratio of atoms in a compound (simplified version of molecular formula)
how to determine the molecular formula from the empirical formula?
calculate the molar mass of the empirical formula
divide the molar mass of the molecular formula by the molar mass of the empirical formula
multiply each subscript by the whole number that resulted from step 2
how to determine the empirical formula from the percent composition?
assume 100 grams of total sample, coverting percentages into grams
divide each number of grams by the element’s atomic mass
divide the numbers from step 2 by whichever number is smaller
if necessary, muiltply all subscripts by an integer to ensure that the smallest whole number ratio is obtained
steps to balance a chemical reaction
identify each element and its quantity for both reactants and products
choose an element on each side to compare the quantities
add a coefficient to balance the elements
repeat until all elements have been balanced
now compare the quanitites of hydrogen on each side
check to see if the chemical equation is balanced by checking if the number of atoms of each element type is the same on each side of the equation
limiting reagent
reagent that determines how much product is formed and is completely used up in a reaction → 0 g at end of reaction
excess reagent
leftover reactant that is not completely used up
how to determine the limiting reagent
make sure chemical equation is balanced
convert all given information into moles
pick one of the reactants and determine how much of the other reactant is required to fully react with the reactant initially chosen
if there is excess of the other reactant, then the reaction you chose is the limiting reagent. if there is a shortage, then the other reactant is the limiting reagent.
density
mass per unit volume
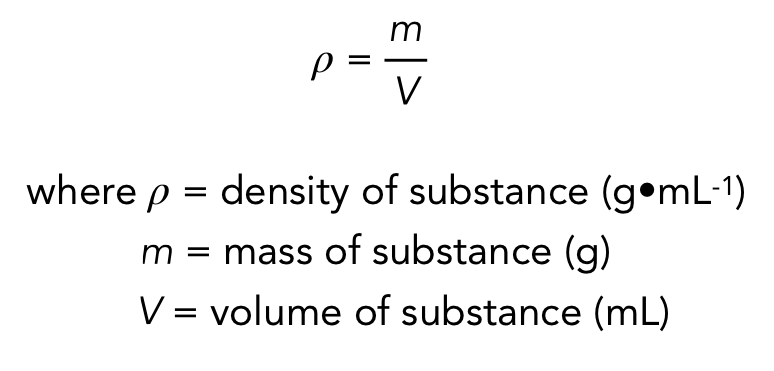
density of water
1 g/mL
*rule of thumb: if an object has a density greater than water, it will sink if placed in it. if it is less, it will float.
under constant temperature and pressure…
density of a pure substance remains constant
actual yield
the real quantity of producr resulting from a chemical reaction
theoretical yield
maximum number of products that you can expect from an ideal chemical reaction
percent yield
measures the efficiency of a chemical reaction
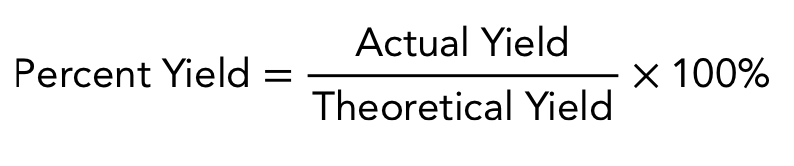
percent yield important notes
full yield is unrealistic → why percent yield is typically < 100%
theoretical yield is calculated using the liniting reagent