Topic 8: Strengthening Mechanisms in Metals
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
any plastic deformation increases the strength of a crystalline material → due to dislocation traffic jam
how does plastic deformation affect the strength of a crystalline material
smaller grains → stronger
bigger grains → weaker
how does grain size affect strength
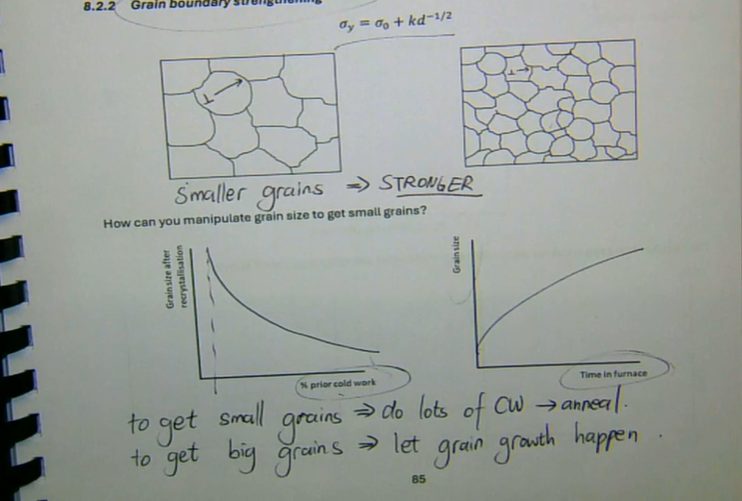
to get small grains → do lots of CW → anneal
to get big grains → let grain growth happen
how can you manipulate grain size to get small grains
if dislocation can move easily → weak and ductile material
to strengthen material → stop dislocation movements
what is the rule of thumb for strengthening mechanisms (for metals)
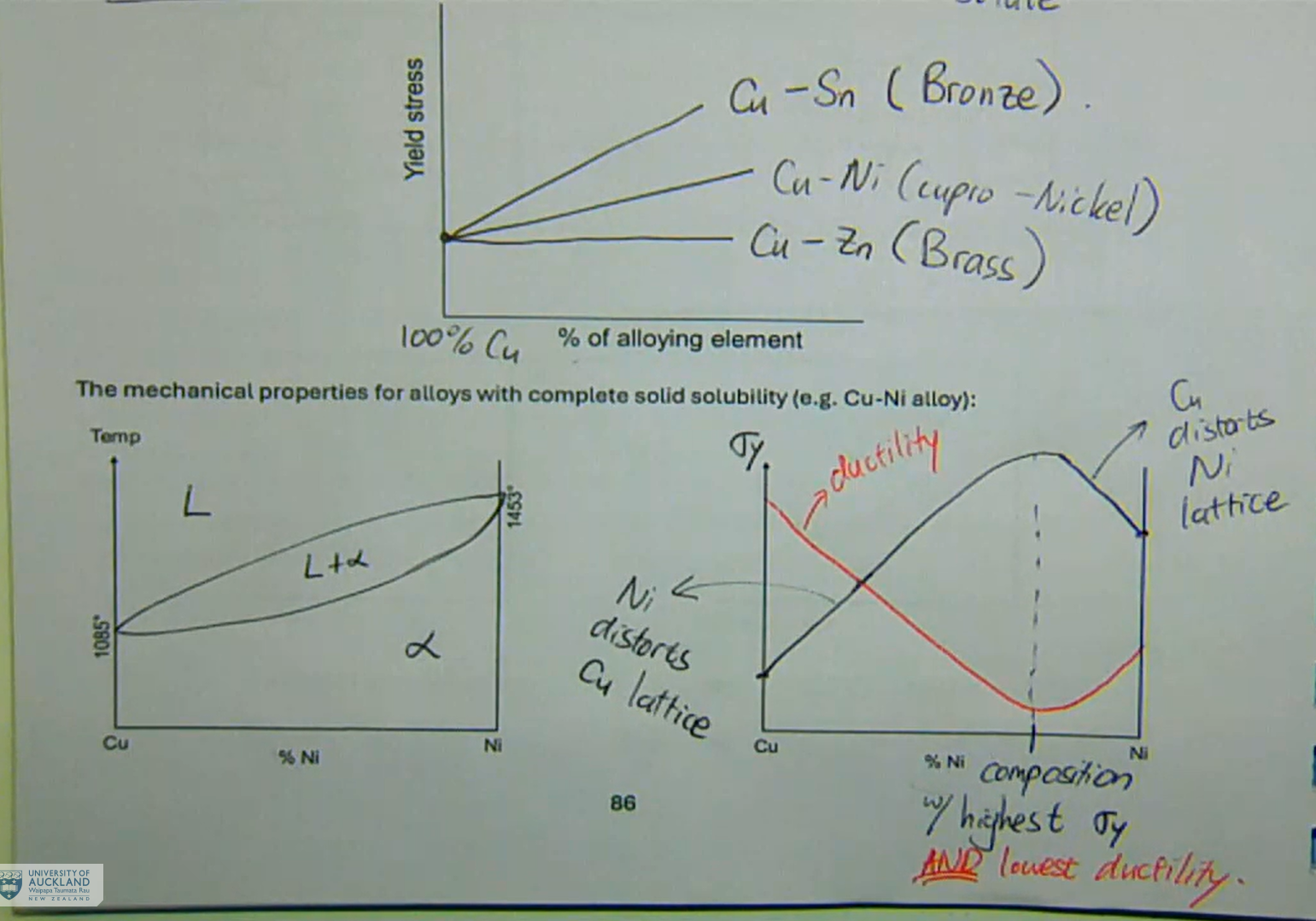
solid solutions can be substitutional or interstitial
atoms of different elements will have different sizes
when atoms of another element are inserted into a crystal lattice, there will be some distortion
smaller solute → greater distortion
the electrical resistivity of the alloy would also change with differing amounts of alloying component
max lattice distortion → max resistivity
solid-solution strengthening
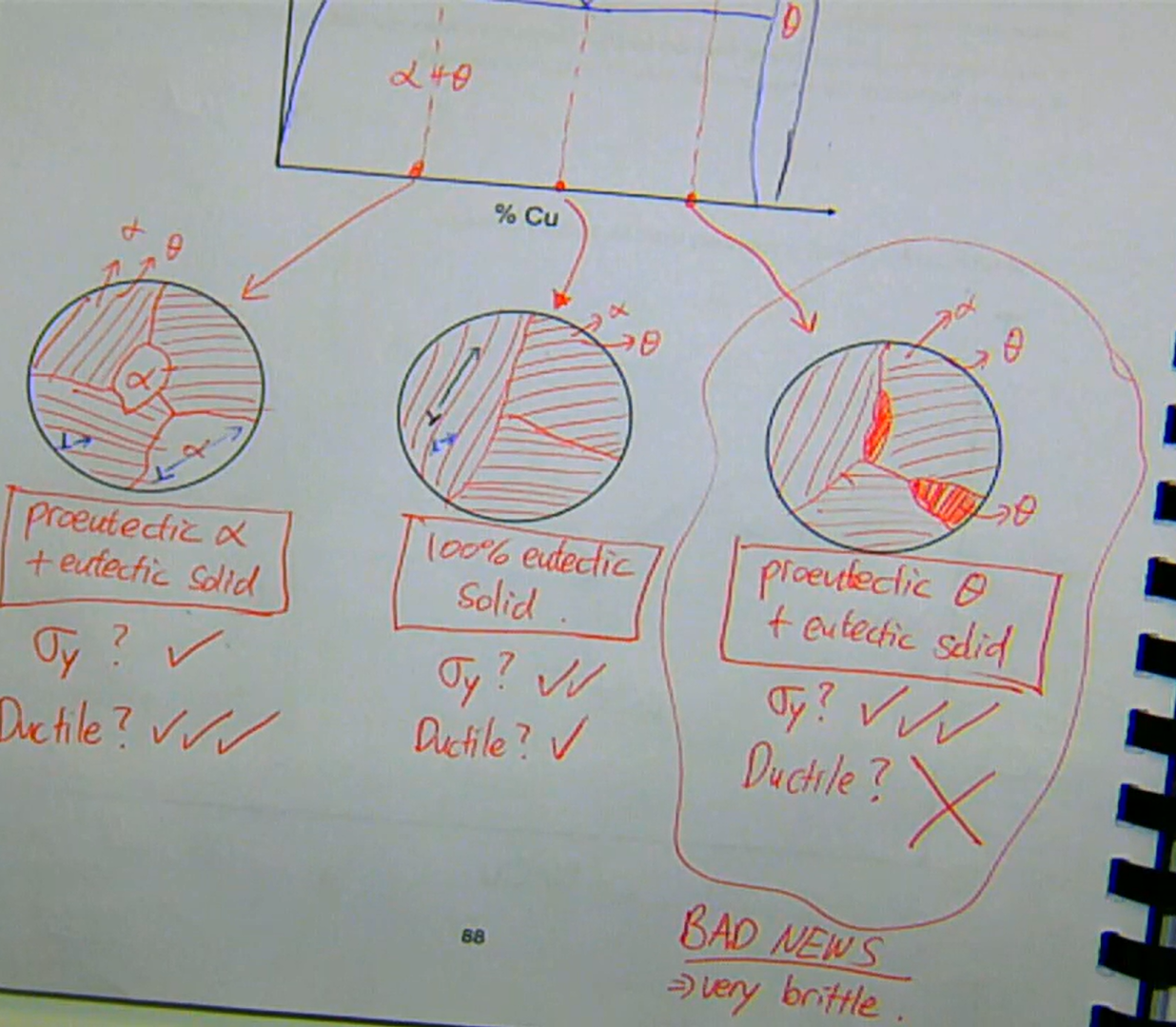
any boundary can inhibit dislocation movement in the crystal lattice
a boundary between two phases (phase boundary) will also inhibit dislocation movement
if you understand how the phase diagram for that alloy works, then you can choose an alloy to maximise the number of boundaries to maximise its strength
to maximise σy → increase % eutectic solid
in the eutectic system, there are only two solid phases of interest:
α → FCC
→ weak and ductileθ → BCT
→ strong and brittle
multiphase strengthening
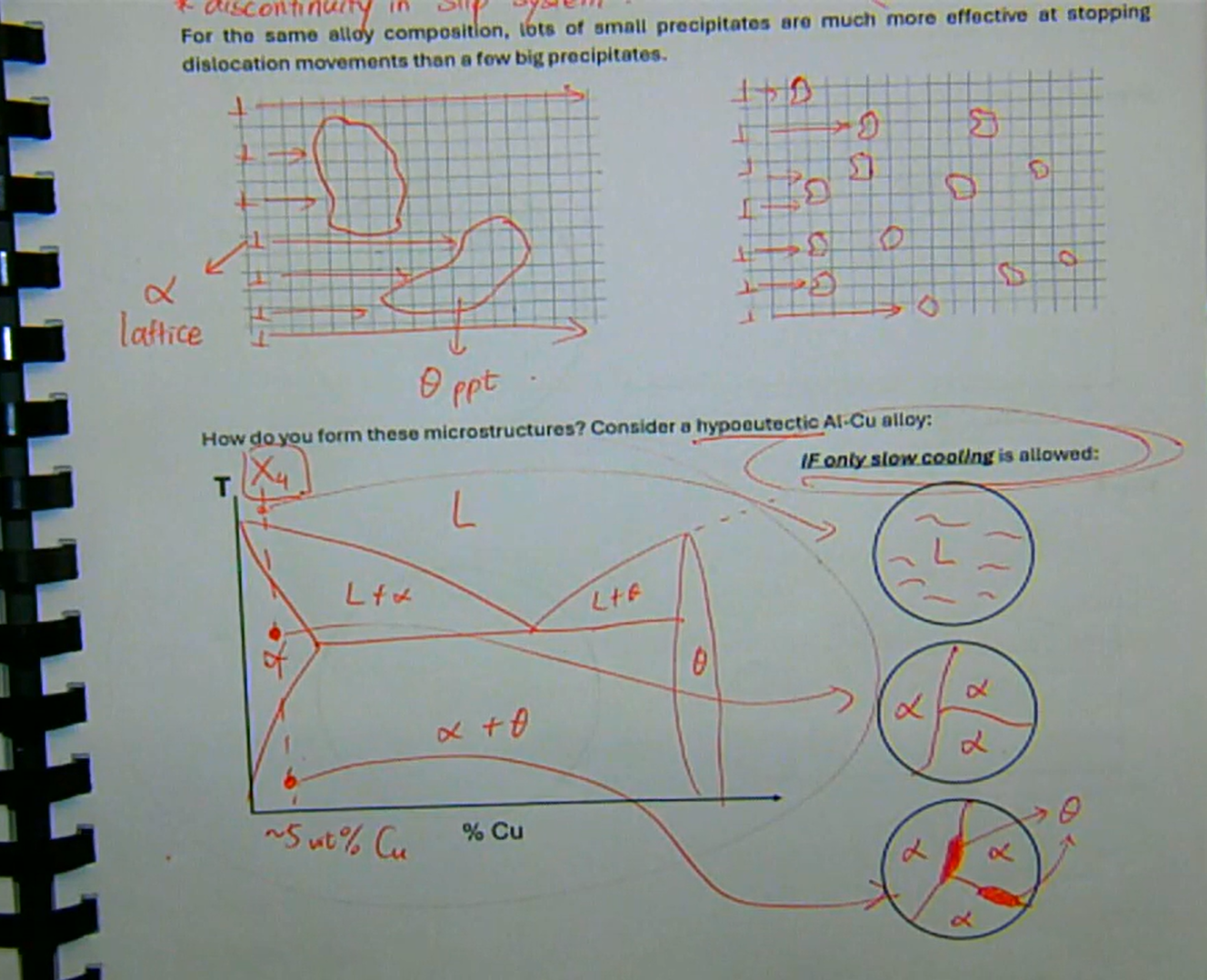
you can further increase the number of boundaries to stop dislocation movements
this is done by having lots of small hard phases (ppts) in a ductile matrix
there are two mechanisms by which precipitates can inhibit dislocation movement and strengthen the alloy:
1. discontinuity in slip system
2. distorting parent lattice
for the same alloy composition, lots of small precipitates are much more effective at stopping dislocation movements than a few big precipitates
these microstructures are formed only if slow cooling is allowed
dispersion strengthening/age hardening/precipitation hardening
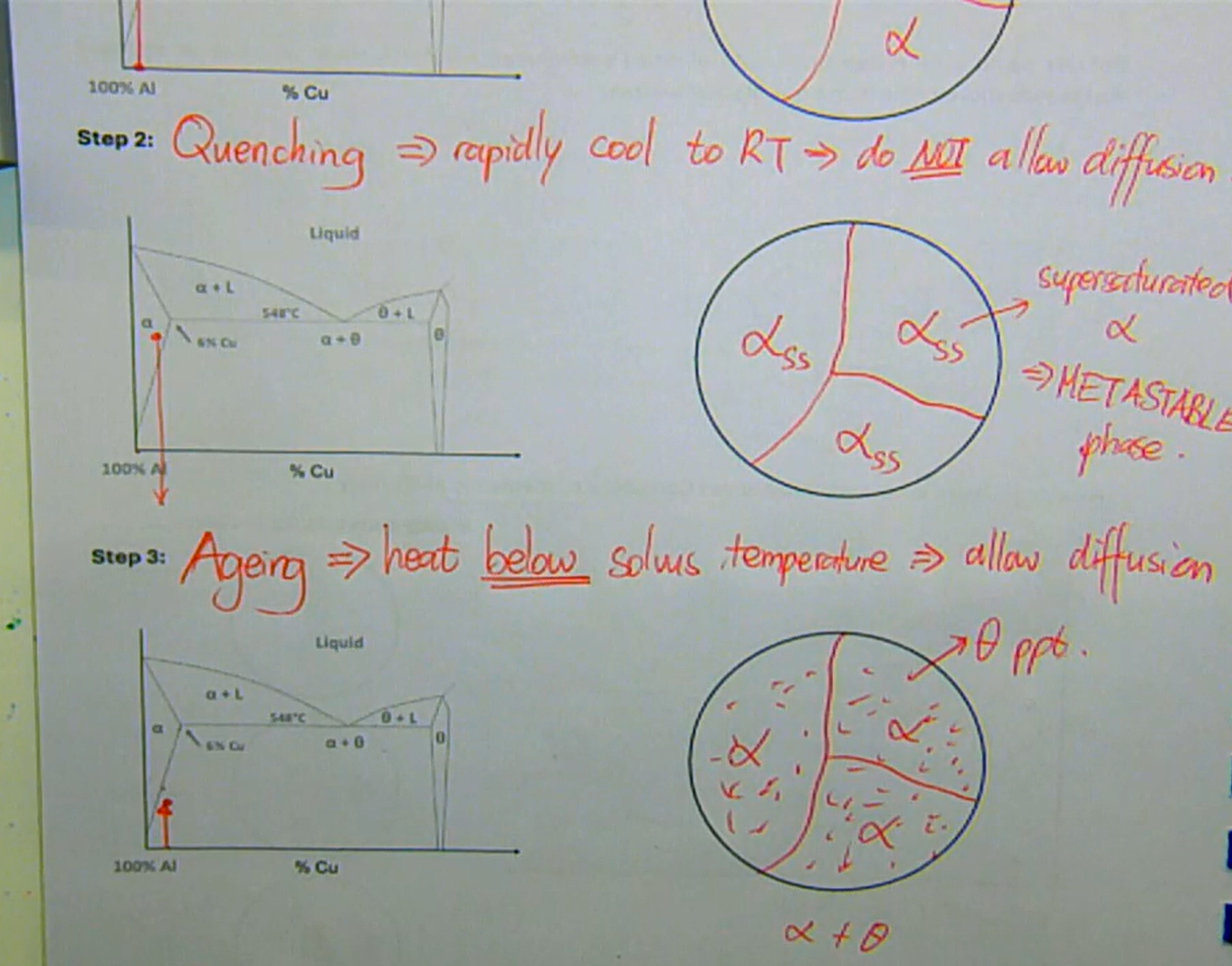
solution heat treatment → heat alloy above solvus to dissolve all Cu atoms
quenching → rapidly cool to RT → do not allow diffusion
forms super saturated α
this is known as the metastable phase
aging → heat below solvus temperature → allows diffusion
what are the three stages of heat treatment used for preparing small precipitates dispersed in another phase
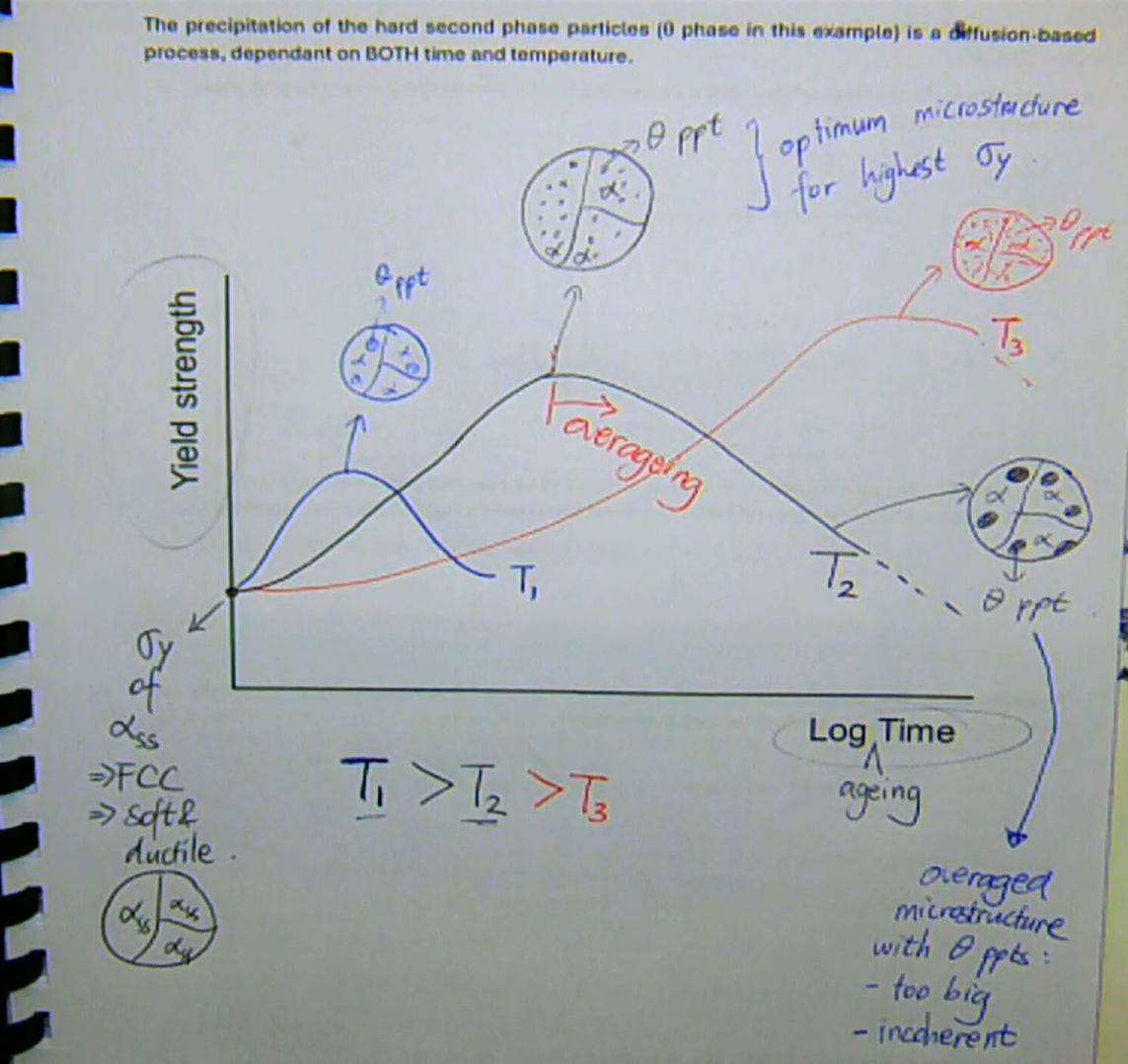
the precipitation of the second phase particles is a diffusion-based process, dependent on BOTH time and temperature
higher aging T (below solvus)
→ less time to reach max σy
→ lower max possible σy (and vice versa)
yield-strength - log(aging time) graph
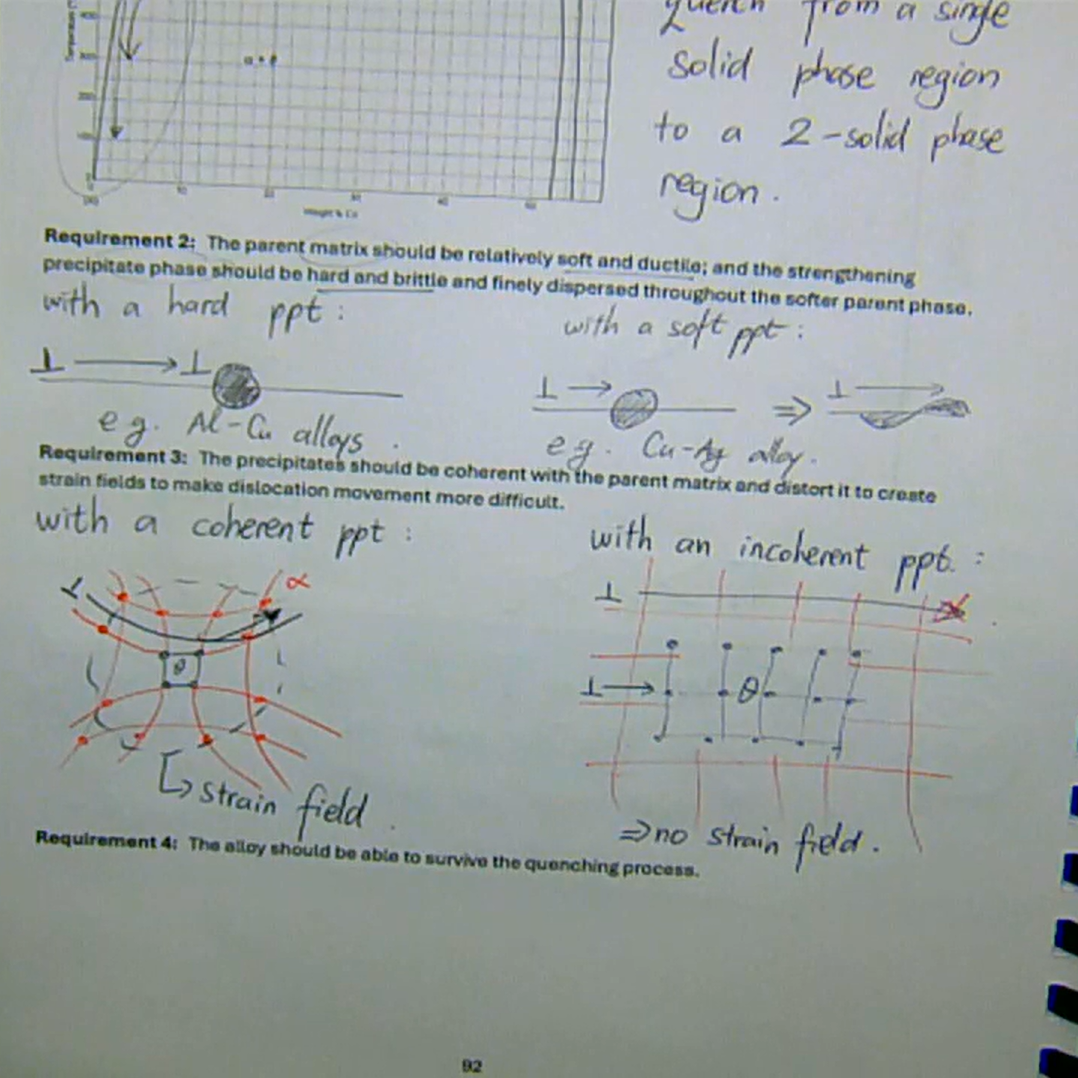
phase diagram must show decreasing solid solubility of the strengthening phase with decreasing temperature
must be able to quench from a single solid phase region to a 2-solid phase region
the parent matrix should be relatively soft and ductile; and the strengthening precipitate phase should hard and brittle and finely dispersed throughout the softer parent phase
the ppts should be coherent with the parent matrix and distort it to create strain fields to make dislocation more difficult
the alloy should be able to survive the quenching process
sudden T change → thermal shock → can cause shape distortions and cracks
what are the four basic requirements before an alloy can be age-hardened
they can not be welded because they will form hard and brittle θ at joints
overage
options for joining:
rivets
screws
glue
why can’t aluminium-based age-hardening alloys be welded together