Chem Prac G
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Describe planning experiment to study the effect of temperature on solubility using gravimetric analysis
Measure 100 cm3 of deionised water using a measuring cylinder into a clean and dry conical flask
Place the conical flask in a thermostatically controlled water bath maintained at 10 °C and use a thermometer to monitor the temperature of the solution inside the conical flask
Using a weighing balance, weigh accurately about 5.00 g of solid Ca(OH)2
Once the temperature inside the conical flask (or beaker) reaches 10 °C, transfer the contents from the weighing bottle into the conical flask
Using a glass rod, stir the mixture
Then leave the mixture to stand over a period of 30 min for equilibrium to be established
Weigh a dry evaporating dish.
Quickly filter the reaction mixture and collect the filtrate.
Pipette 10.0 cm3 of the filtrate into the evaporating dish and evaporate it to dryness.
Cool, weigh and record the mass of the dish with the residue.
Repeat steps 1 to 9 four more times at temperatures of 20 ºC, 30 ºC, 40 ºC, 50 ºC
Draw planning experiment to study the effect of temperature on solubility using gravimetric analysis
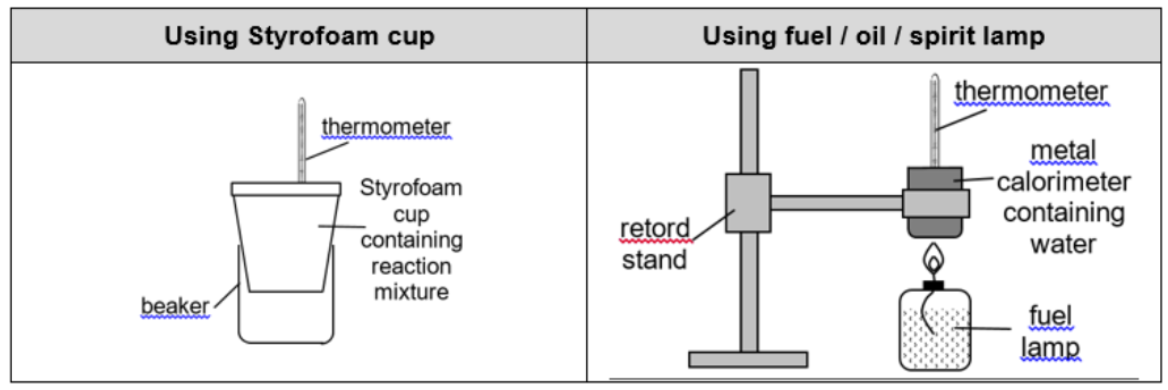
Describe experiment for using gas syringe
Using a weighing balance, accurately weigh XXX g of the sample in a boiling tube.
Record the initial reading of the syringe.
Start heating the solid gently then strongly until the plunger of the syringe does not move.
Allow time for the volume of the gas to equilibrate to room temperature and pressure, or until no more movement of piston is observed.
Record the final reading of the syringe.
Repeat the experiment to improve reliability of results
NOTE:
Include the pre–calculated mass of substance to be used so that the total volume of gas evolved should be about 50% to 80% of the capacity of gas syringe, measuring cylinder or burette used to collect the gas
Describe experiment for using gas syringe with thistle
Using a weighing balance, weigh accurately about 0.905 g barium sulfite in a weighing bottle.
Record the total mass of the weighing bottle and the sample, m1. Transfer this into a 250 cm3 conical flask. Reweigh the weighing bottle after transfer, and record its mass, m2. The mass of barium sulfite sample used = m1 − m2
Measure the volume of acid used (ensure it is in excess) with burette and record it as Vacid
Measure the maximum volume of gas collected by reading off the graduated gas syringe when the piston stops moving
Record temperature and pressure reading of the collected gas at the end of the collection [using thermometer and barometer respectively]
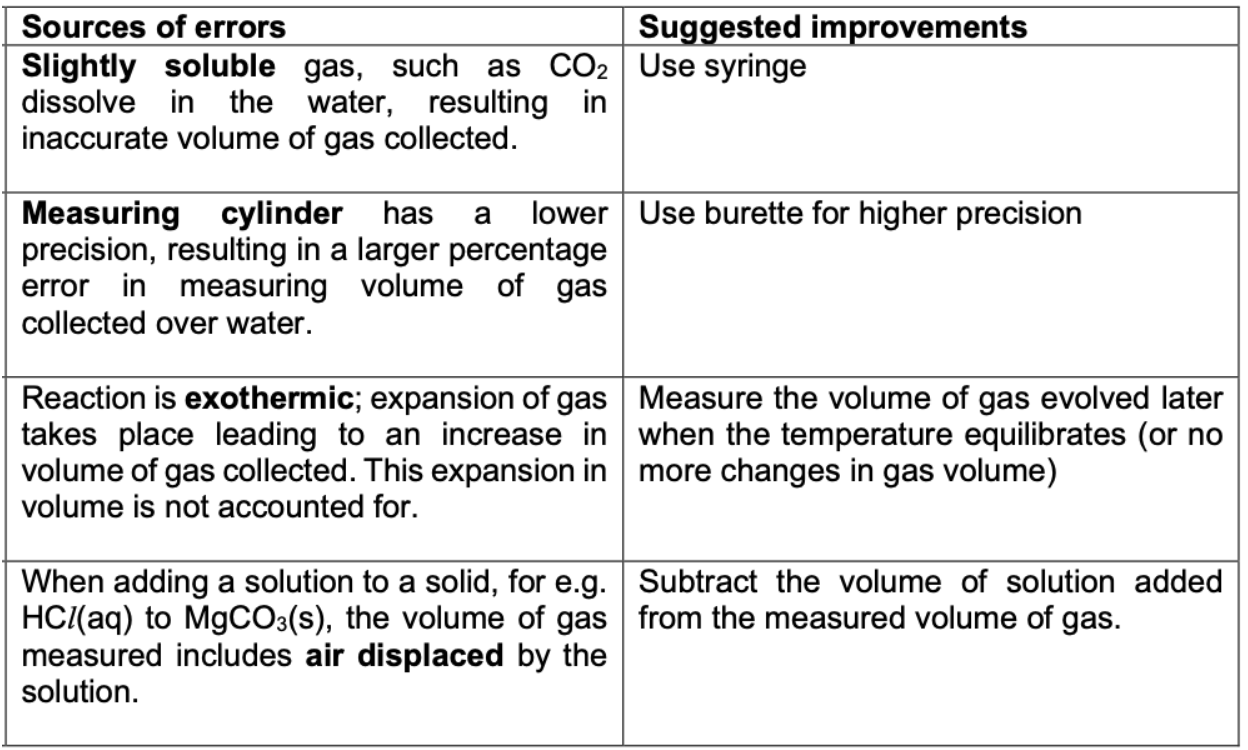
Describe collecting gas errors
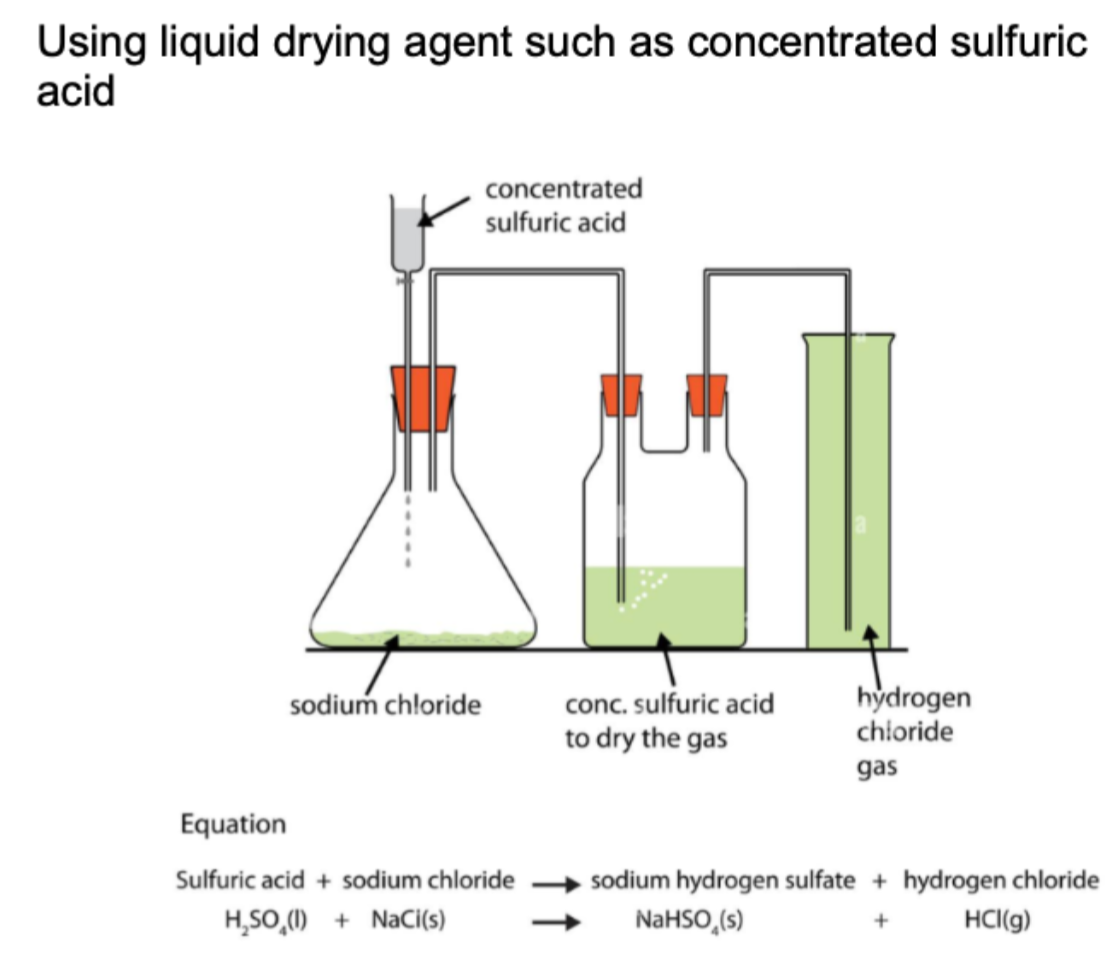
Draw experimental set up for drying agents (H2SO4)
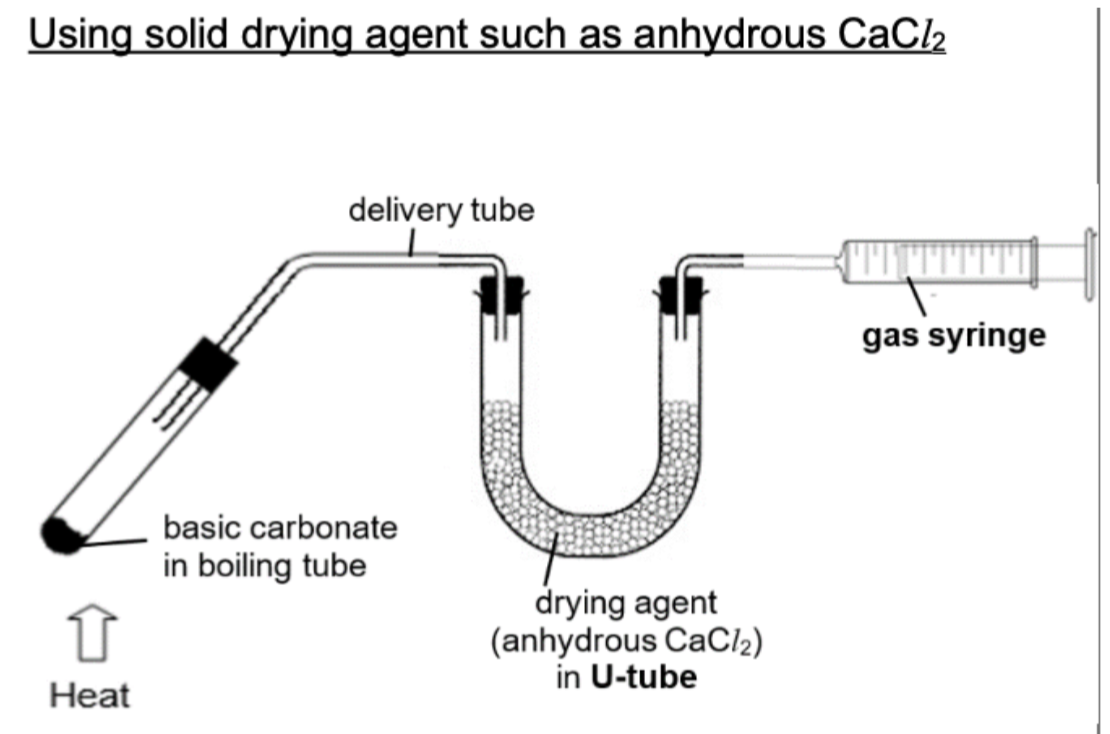
Draw experimental setup for drying agent (anhydrous CaCI2)
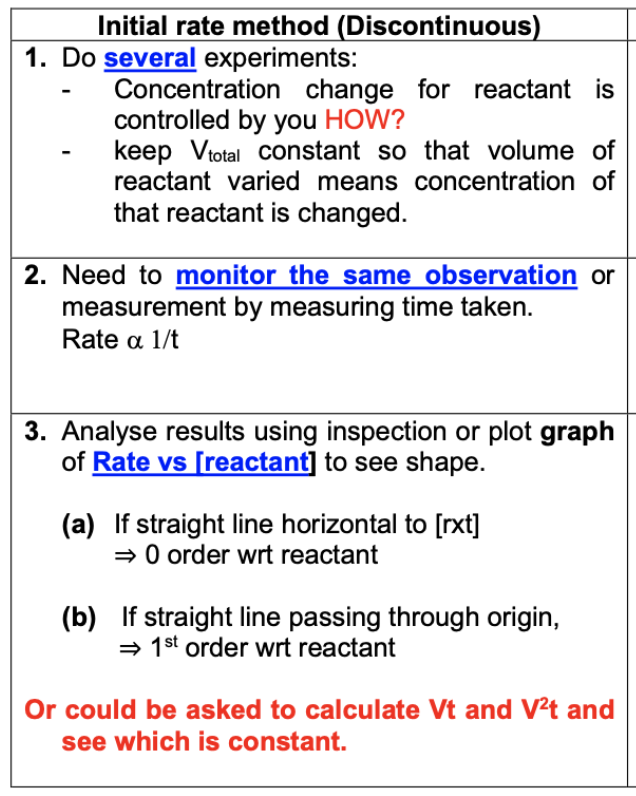
Describe initial rate method (how many experiments, observations, graph to analyse results)
Describe continuous rate method (how many experiments, observations, graph to analyse results)

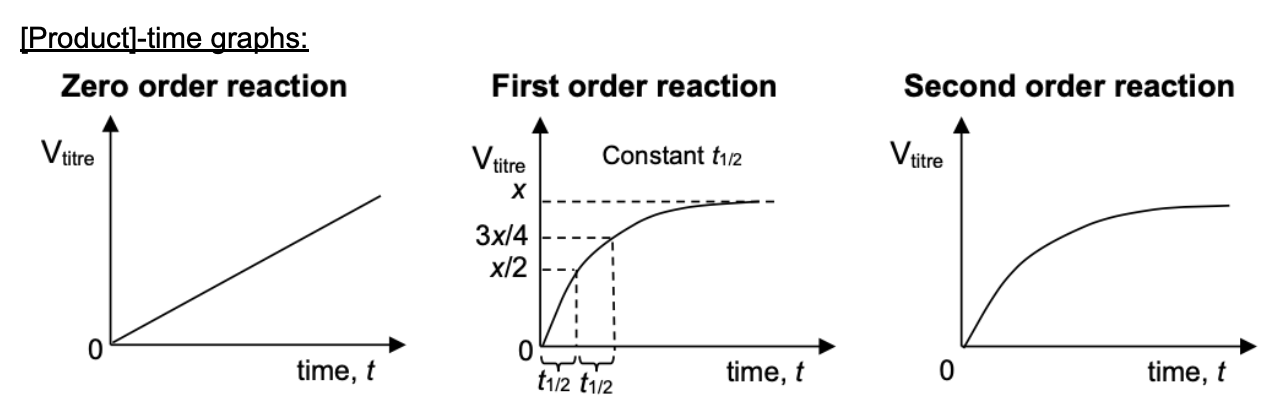
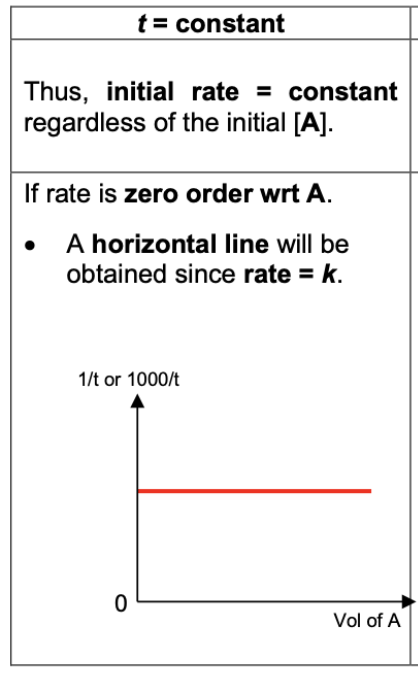
Draw and describe graph for 0 order reaction (1/t against [A])
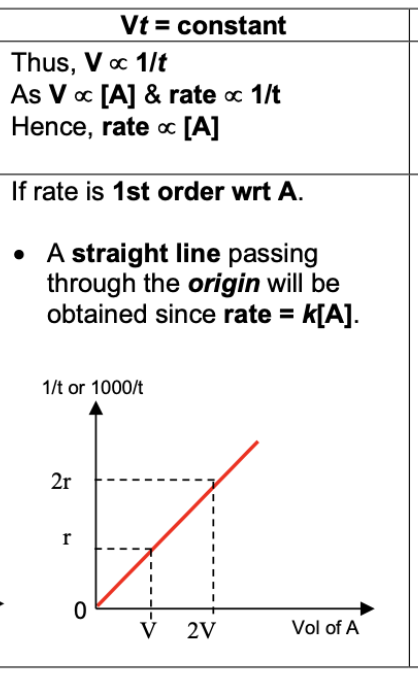
Draw and describe graph for 1 order reaction (1/t against [A])
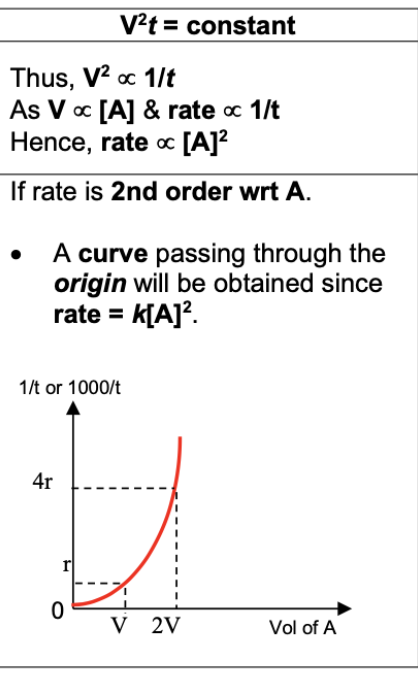
Draw and describe graph for 2 order reaction (1/t against [A])
Describe the planning experiment for continuous method
Only 1 reaction mixture prepared but sampling is done at regular time intervals
Remove samples from the reaction mixture at certain time intervals from the start of the reaction
Quench the samples immediately at the specified time (At 2 minutes, such as adding NaHCO3(s) or adding 100 cm3 of cold water)
To remove the acid catalyst or to slow down the reaction rate through dilution (increase volume) and decrease in temperature
Titrate the sample with a suitable titre to determine the [reactant]left or the [product]formed in the sample
Repeat steps 2 to 4 for different time, t
To determine the order of reaction wrt I2, plot the graph of Volume of titre, Vtitre, used at specified time against time t
Draw [reactant] time graphs for 0, 1, 2 order reaction
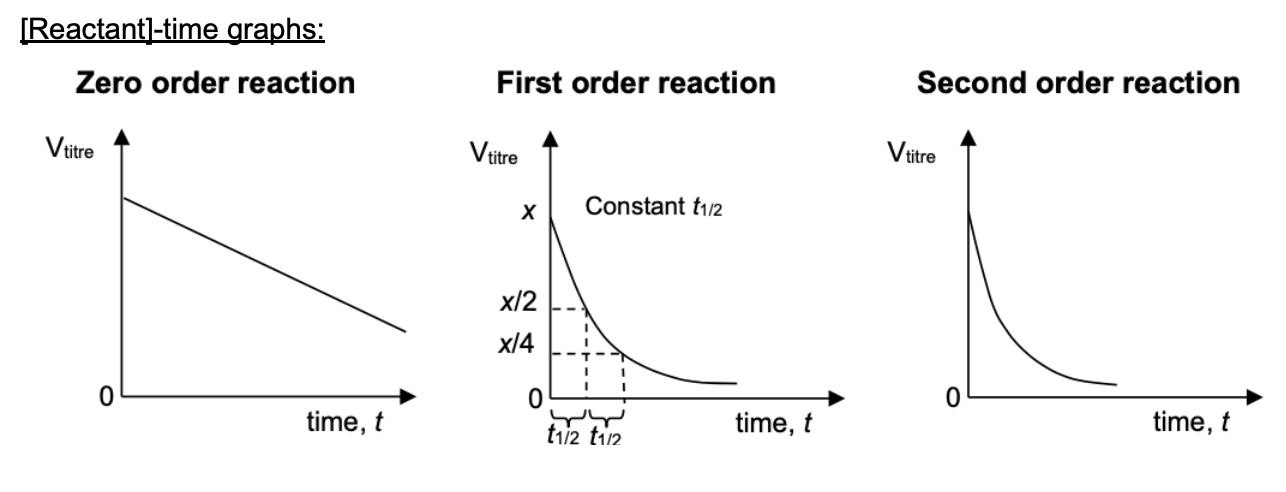
Draw [product] time graphs for 0, 1, 2 order reaction