Chem 10/8
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
How to get number of neutrons
Mass number - proton/atomic number
Atomic number =
Proton
Proton =
Atomic number
How to find mass number
Periodic table (top left number)
How to find atomic number
Periodic table (bottom left, top number)
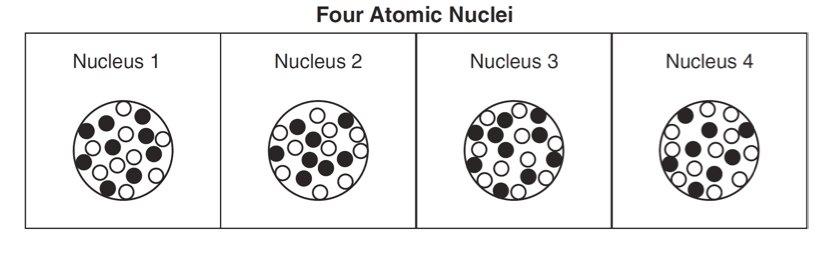
How to find mass number when given atom chart
Proton+ neutron =mass number
Proton in neutral atom =
Electron
How to find now many electrons are in each Shell
Periodic table ( bottom left number )
As the shells get further away from the nucleus
Energy increases
Max fill for electron Shells
2 - 8-18-32
Excited state =
More energy
The lines on the line spectra represents
The energy released as an electron moves from a higher energy state to a lower energy state
Isotopes have different
Mass and neutrons
Isotopes have the same
Symbol, element, atomic number, protons and elections
Murcury-202 , what does 202 represent
The mass number
Neutrons =
Mass number
Why are atoms with different numbers of neutrons still considered to be the same element?
They have identical numbers of protons and electrons, which determine chemical behavior
When the question asks for the charge of the nucleus,
The answer is atomic number
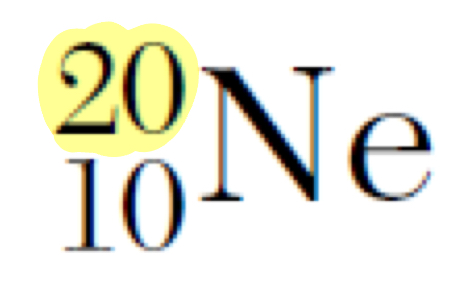
What does the highlighted number represent
Mass
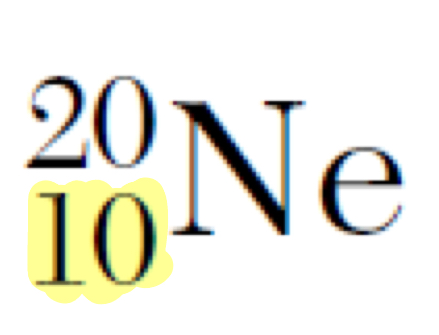
What does the highlighted number represent
Atomic number
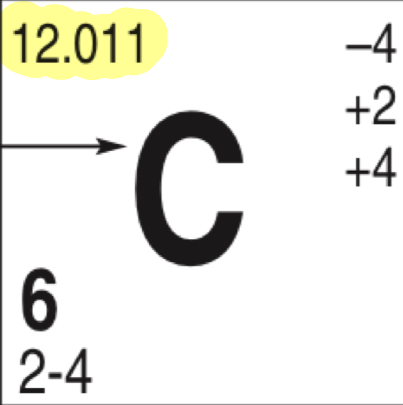
What does the highlighted number represent
Atomic mass/neutrons
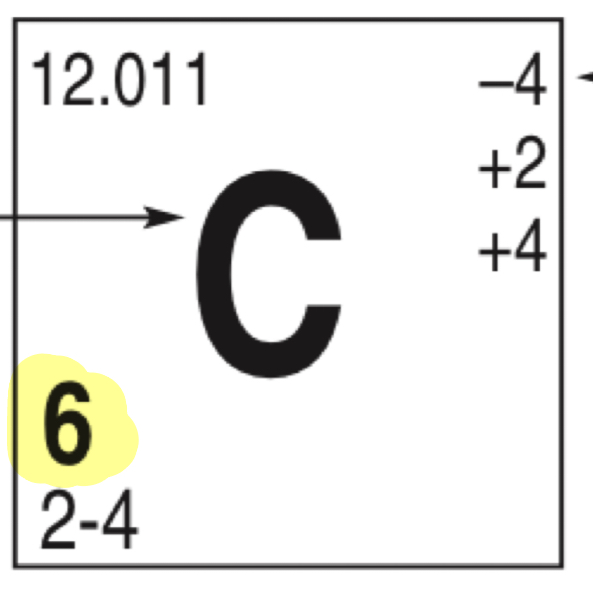
What does the highlighted number represent
Atomic number/protons
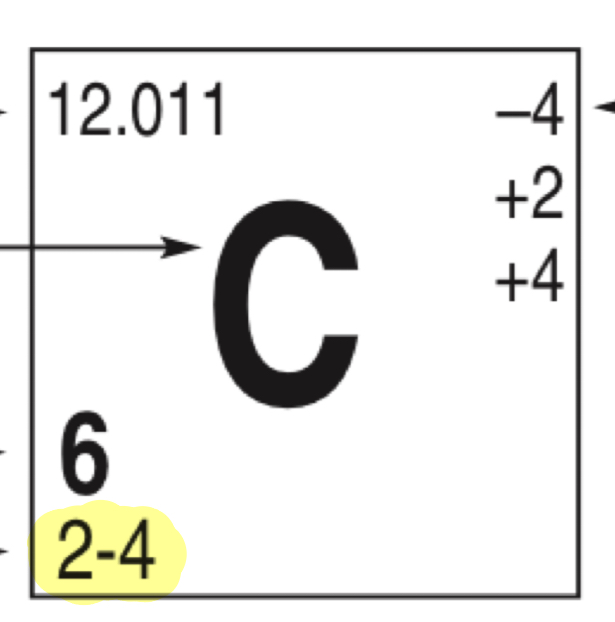
What does the highlighted number represent
Amount of electrons in shells
Ground state
Lowest energy arrangement
Ground state is found
On the periodic table bottom left corner
Excited state
electron absorbs energy and jumps to a higher shell. An inner shell is left partially empty
When electrons fall back to ground state from excited state, they
Emit energy (light/heat)
Charge of a proton
+1
Charge of a neutron
Neutral
Location of proton
Nucleus
Location of neutron
Nucleus
Charge of electron
-1
Electron location
Outside of nucleus
Atomic mass
weighted average of naturally occurring isotopes