Chapter 11- Reactions of Alcohols Organic Chemistry
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Oxidation
Loss of H2
Gain of O, O2 or X2 bonds
Reduction
Loss of O, O2, or X2
Gain of H2 or H
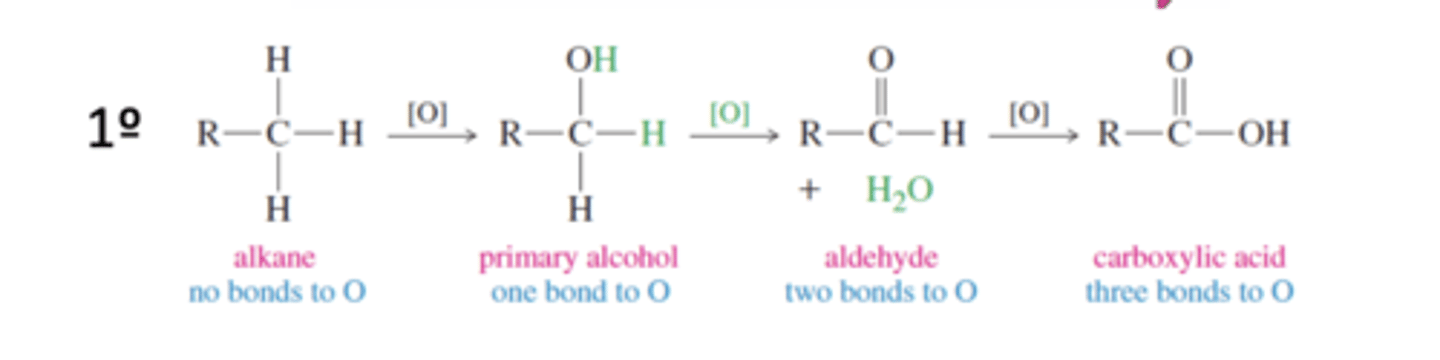
Primary Alcohol Oxidation States
Alkane → 1° Alcohol → Aldehyde → Carboxylic Acid
Secondary Alcohol Oxidation States
Alkane → 2° Alcohol → Ketone
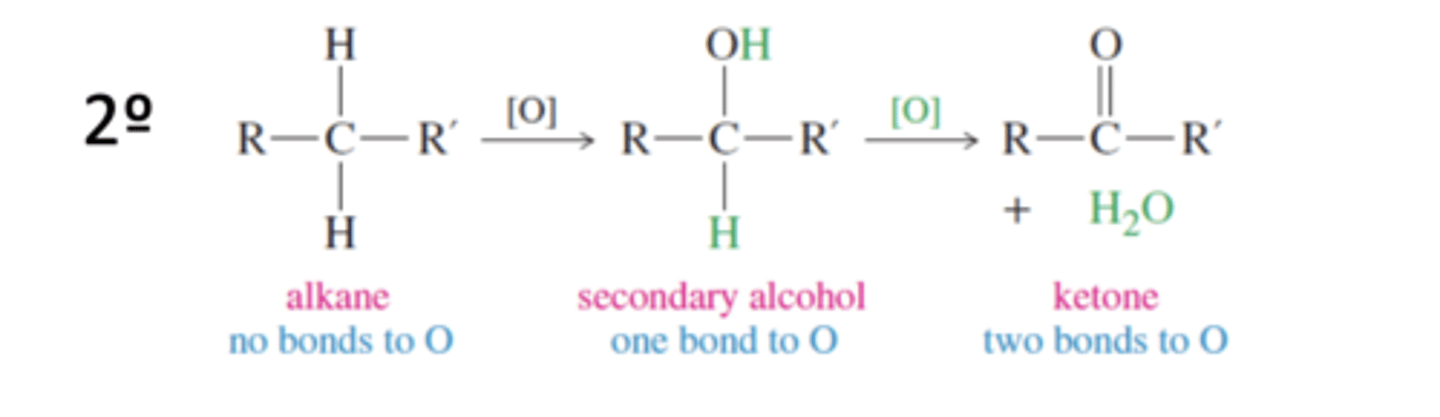
Tertiary Alcohol Oxidation States
Alkane → 3° Alcohol
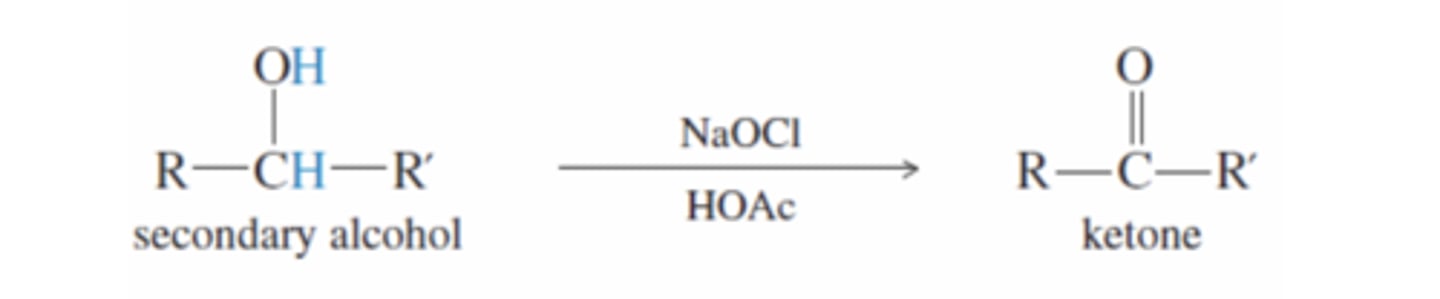
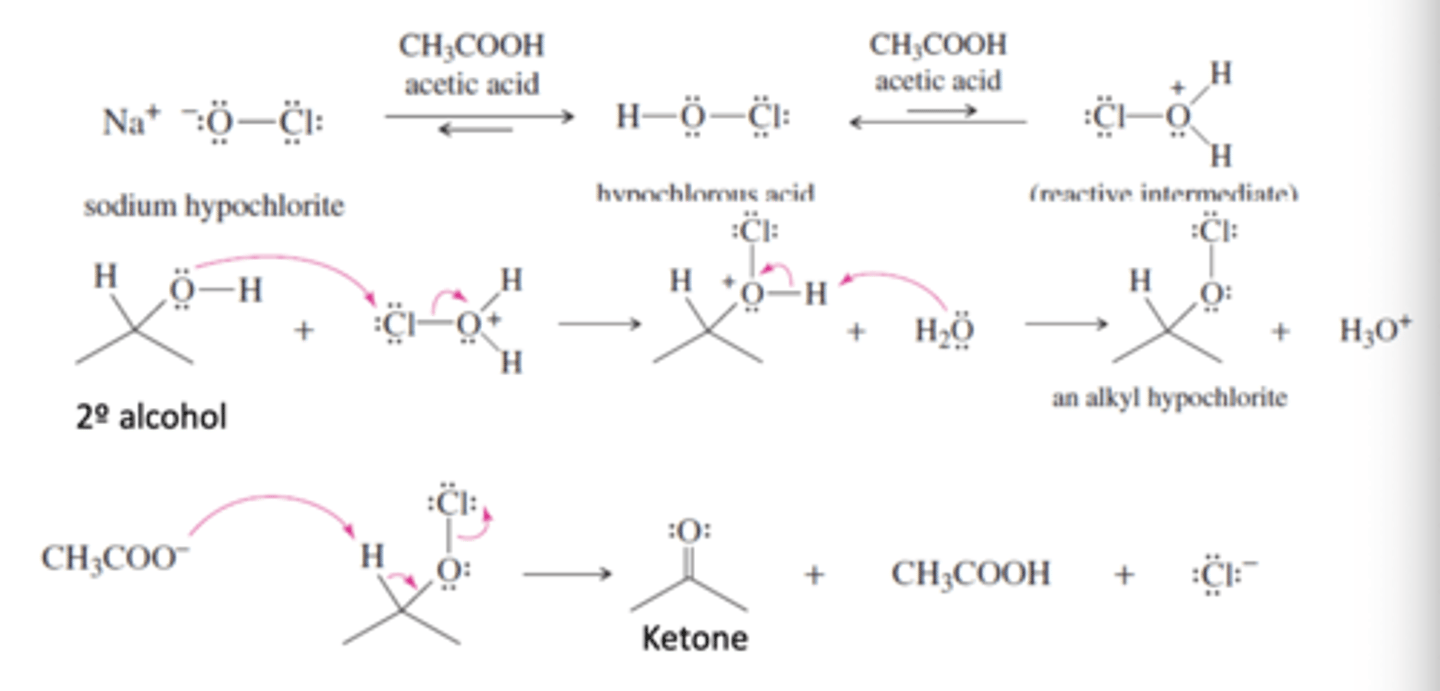
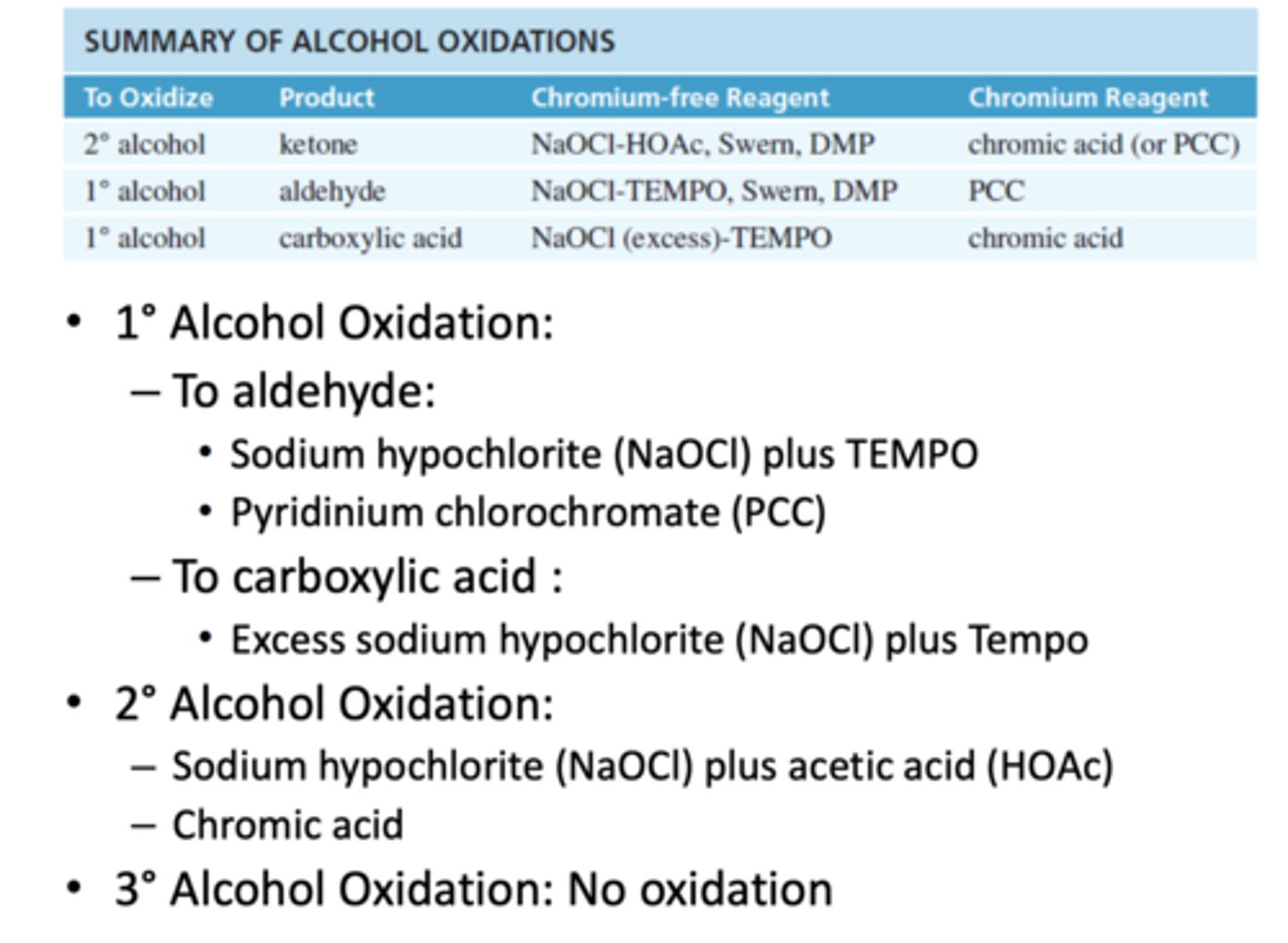
Sodium Hypochlorite (NaOCl) in the presence of acetic acid
-Oxidizes 2° Alcohols to ketones
-Acetic acid protonates NaOCL to hypochlorous acid and then activates hypochlorous acid to become a strong electrophile
-Oxygen in alcohol attacks the Cl
-Acetate ion removes a proton and chlorine leaves
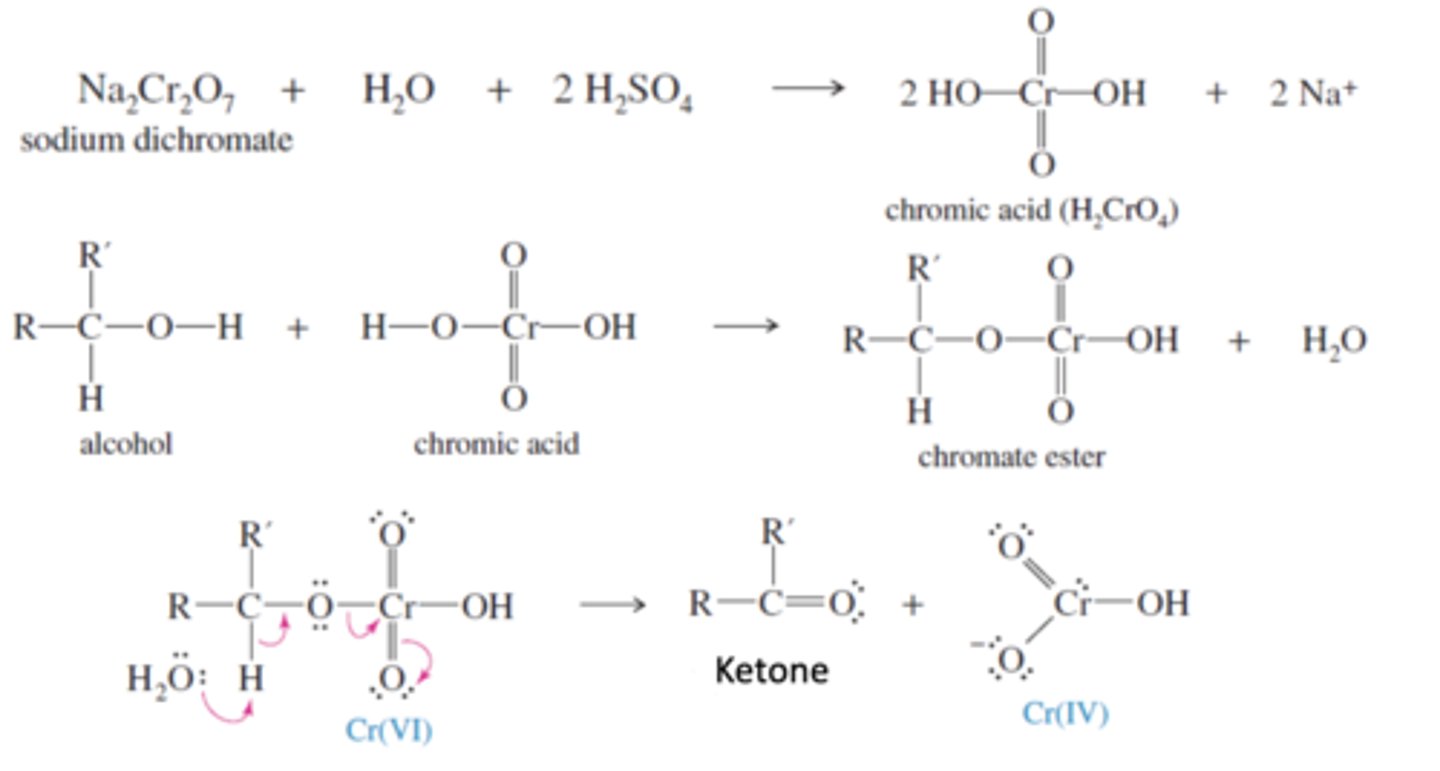
Chromic Acid (H2CrO4)= Sodium dichromate (Na2Cr2O7) + Sulfuric Acid (H2SO4) and Water
-Oxidizes 2° Alcohols to ketones
-Alcohol oxygen attacks chromic acid oxygen to create a chromic ester and water
-Water protonates Chromate ester to form water as a leaving group to create a ketone and Chromic acid
Sodium hypochlorite (NaOCl) plus TEMPO (stable free radical)
-Oxidizes 1° Alcohol to an aldehyde
-When sodium hypochlorite plus TEMPO is in excess it oxidizes to carboxylic acid
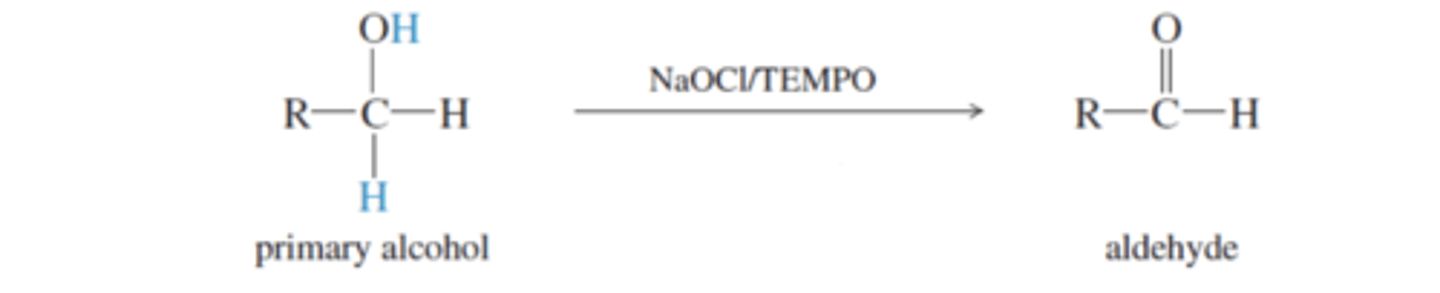
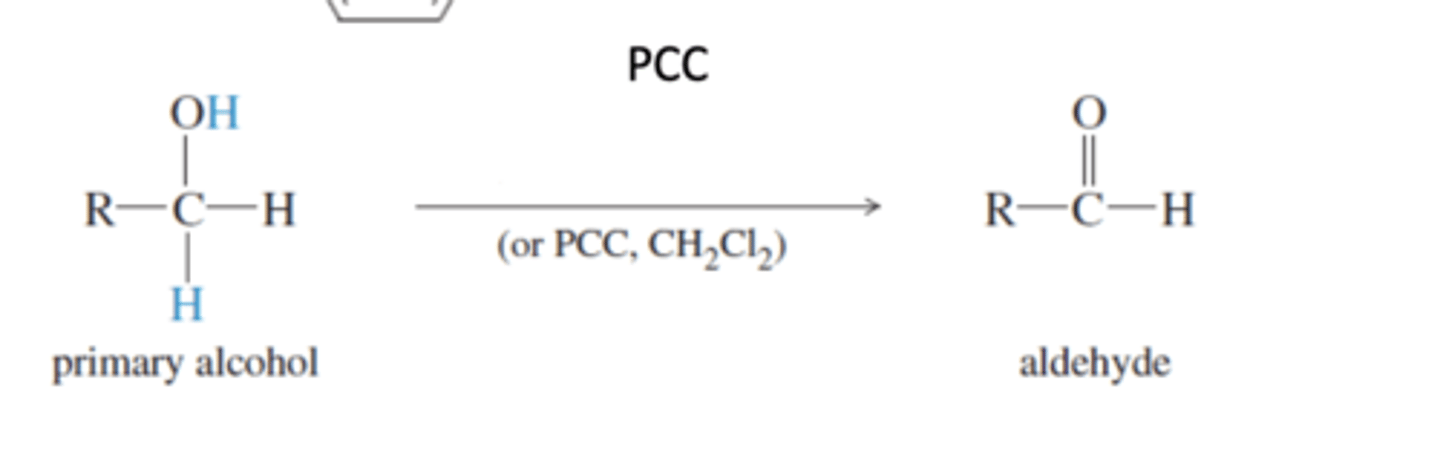
Pyridinium Chlorochromate (PCC) (usually in dichloromethane [CH2Cl2])
-Oxidizes 1° Alcohols to an aldehyde
Summarize Alcohol Oxidations
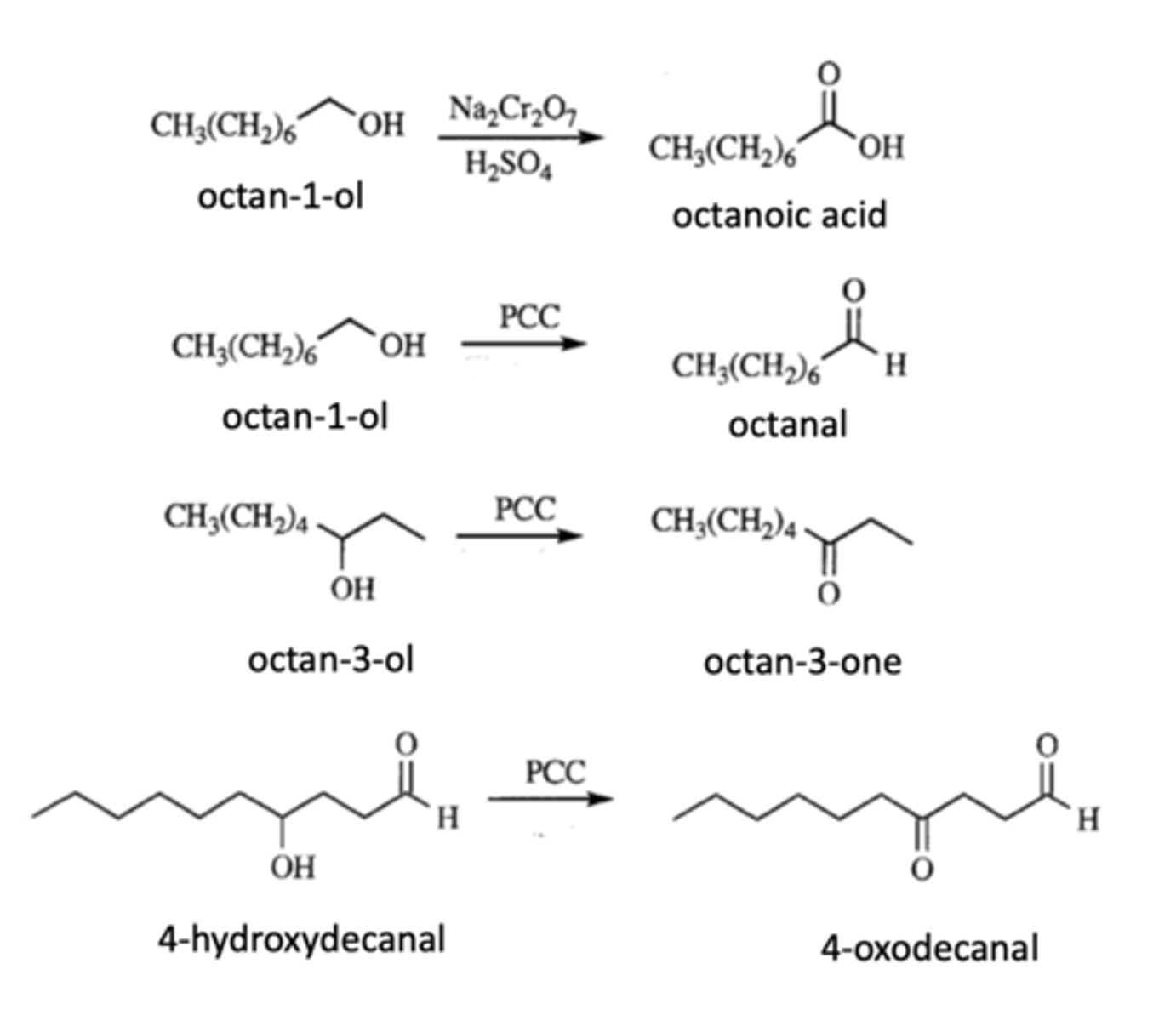
What does it oxidize to?
a) Octan-1-ol with sodium dichromate in the presence of sulfuric acid
b)Octan-1-ol with PCC
c) octan-3-ol with PCC
d) 4-Hydroxydecanal with PCC
How to oxidize a 3° alcohol?
Nothing, it has no oxidation
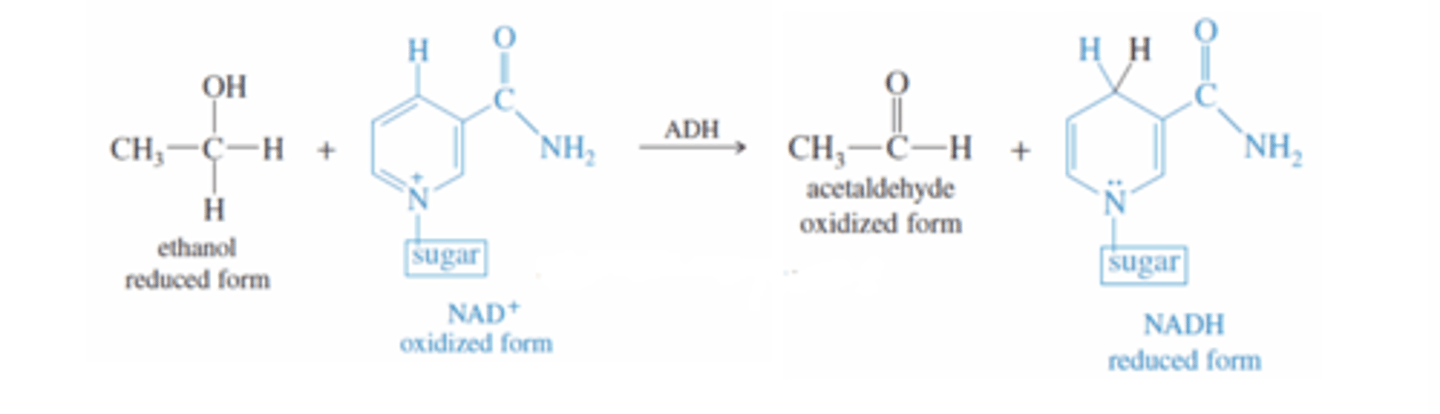
Ethanol + ADH
-ADH catalyzes oxidation of ethanol by NAD to an aldehyde
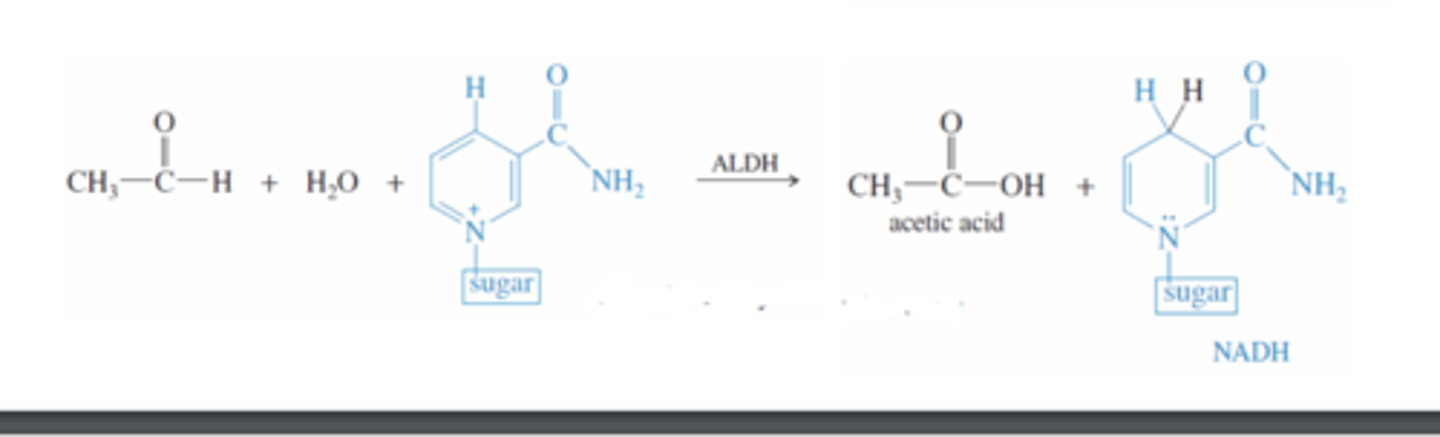
Ethanol + ALDH
-ALDH catalyzes the oxidation of ethanol by NAD to carboxylic acid (acetic acid)
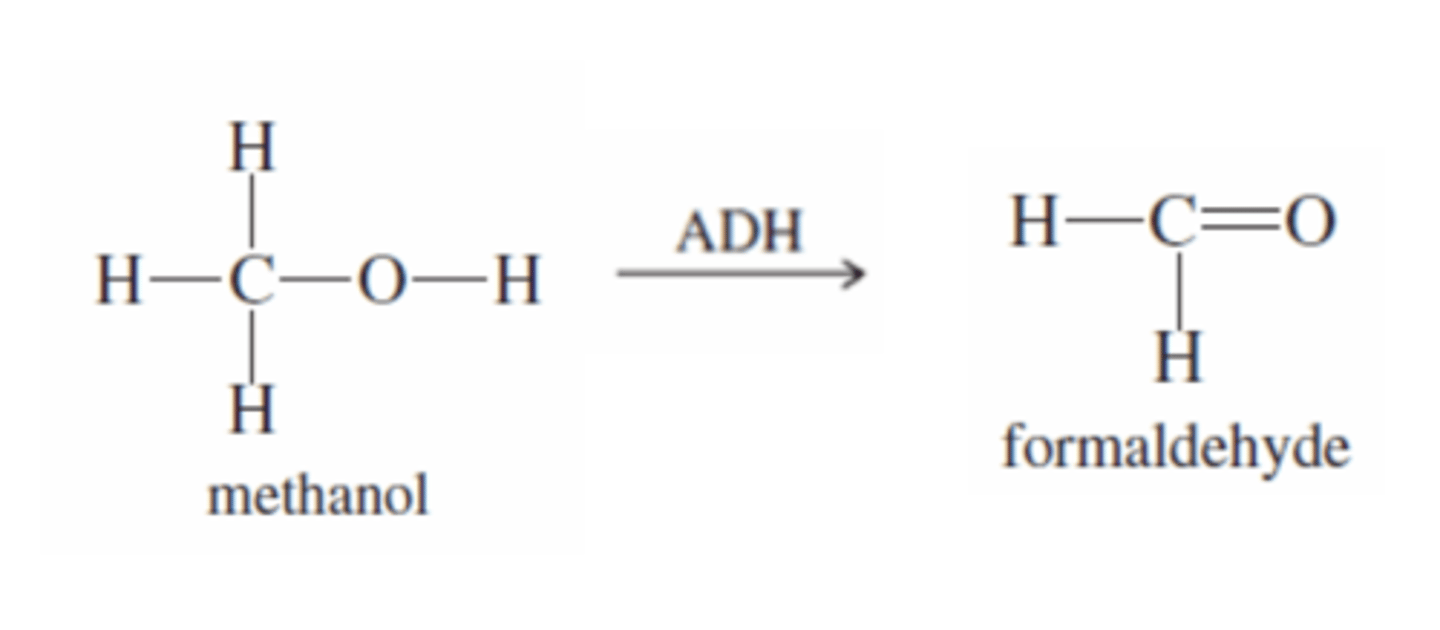
Methanol + ADH
-ADH catalyzes oxidation of methanol to formaldehyde (Aldehyde)
-TOXIC
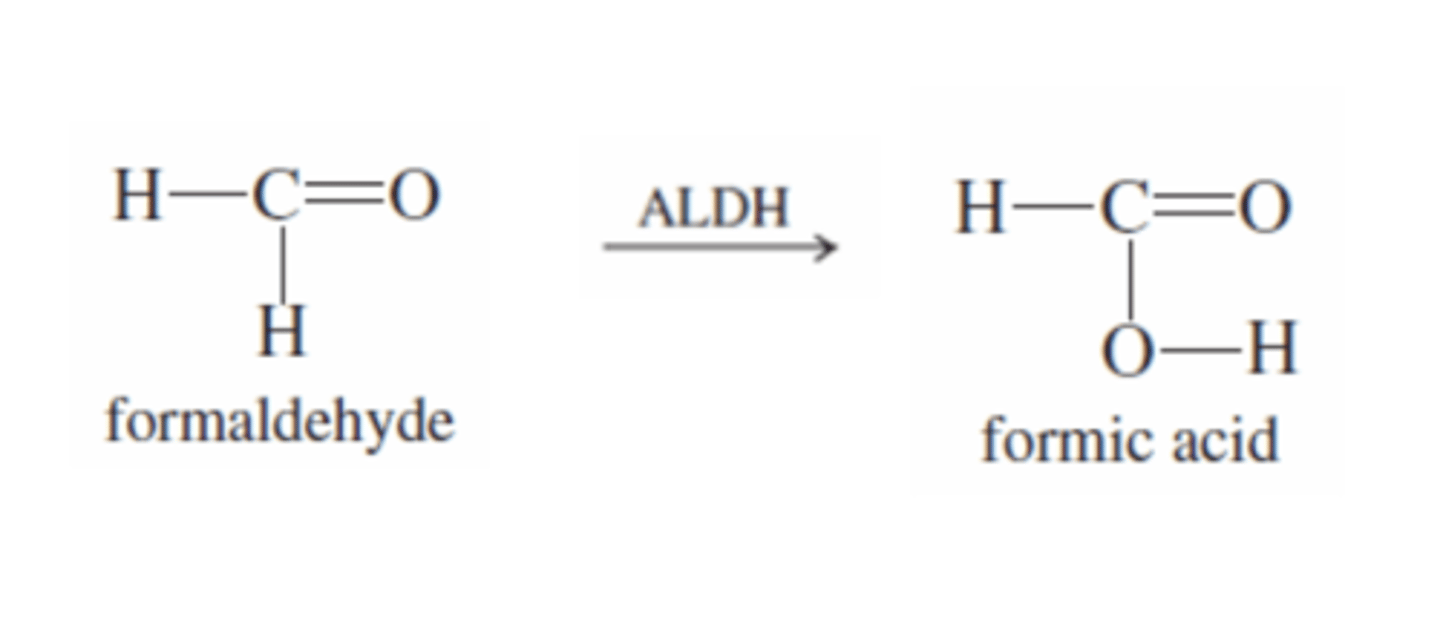
Methanol + ALDH
-ALDH catalyzes the oxidation of formaldehyde to formic acid (Carboxylic acid)
-EVEN MORE TOXIC
How is methanol poisoning treated?
Treated by ethanol intravenous infusion because the ethanol will compete for ADH and ALDH to slow/stop the oxidation of methanol into toxic substances
When can Alcohol act as a nucleophile?
1) Alcohol can react as a weak nucleophile when there is a strong electrophile to attack
2) Alcohol can be converted to a strong nucleophile by forming its alkoxide ion to attack a weak electrophile
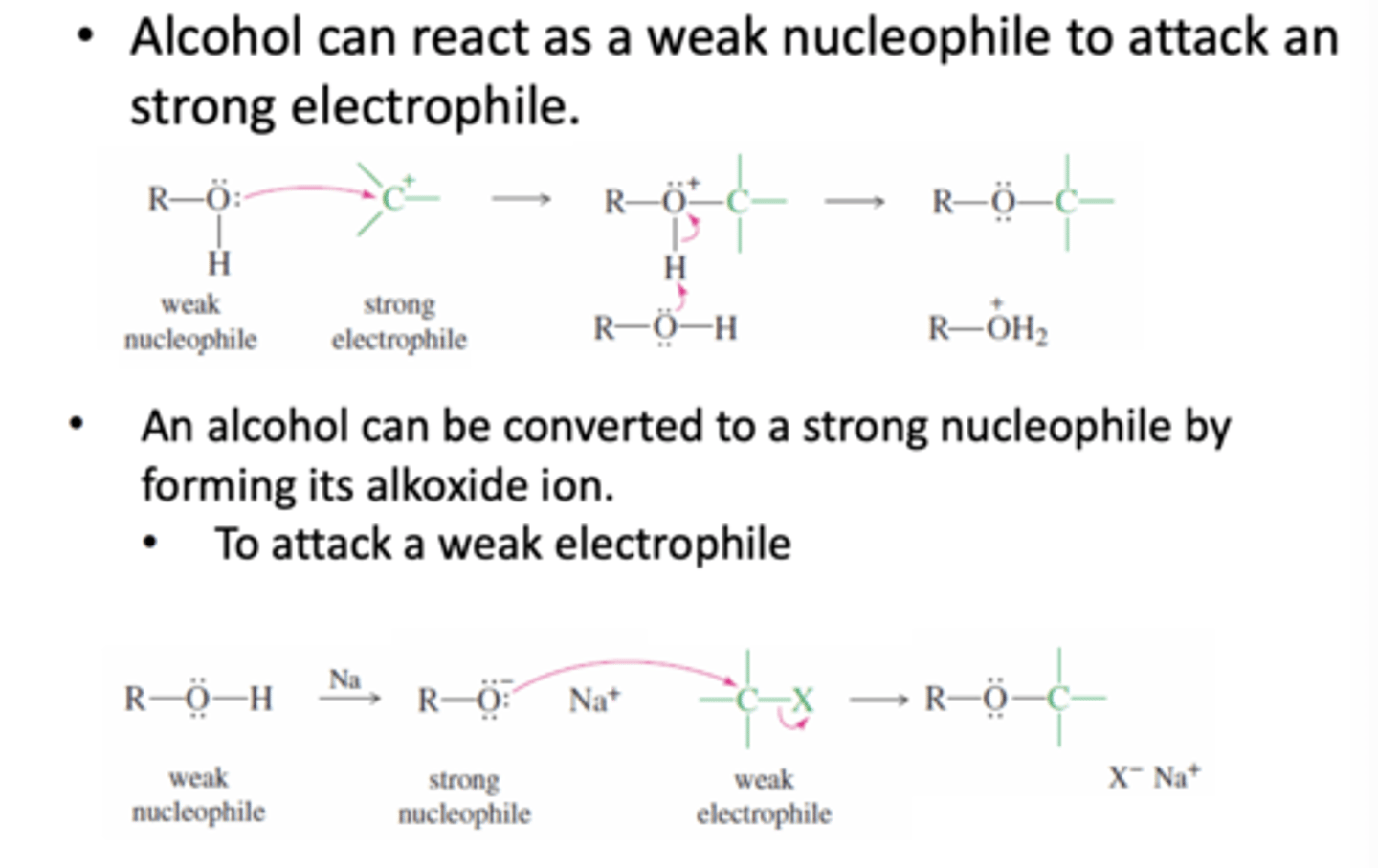
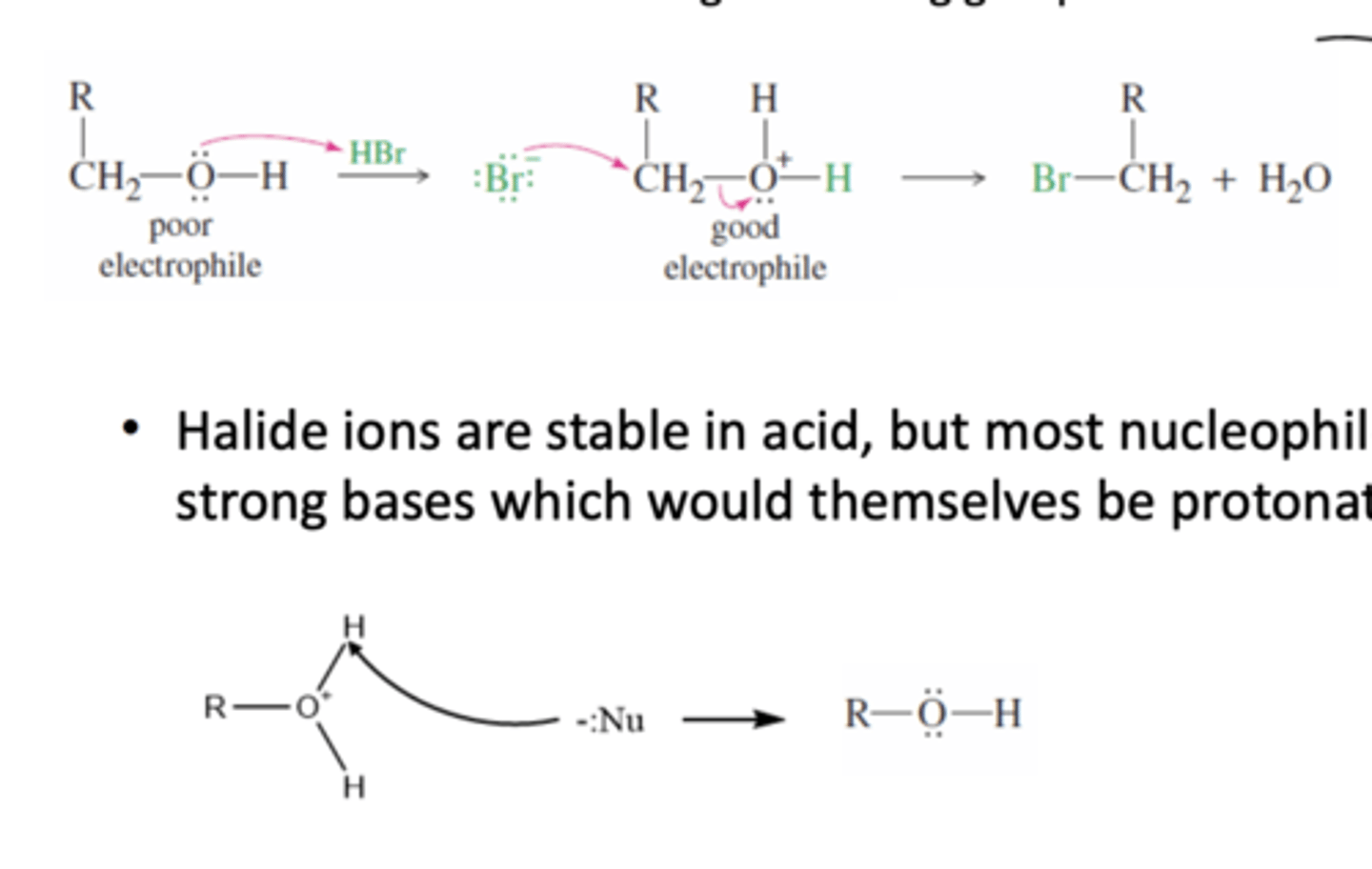
When can alcohol act as an electrophile?
1) Protonation can make alcohol a good leaving group, hence a good electrophile
2) In a Aprotic solution
→ Alcohol is weak as an electrophile because the hydroxy group is a poor leaving group
→ Cannot in a protic solution because Halide ions, which become the nucleophile after protonating the alcohol, are strong bases which would themselves be protonated
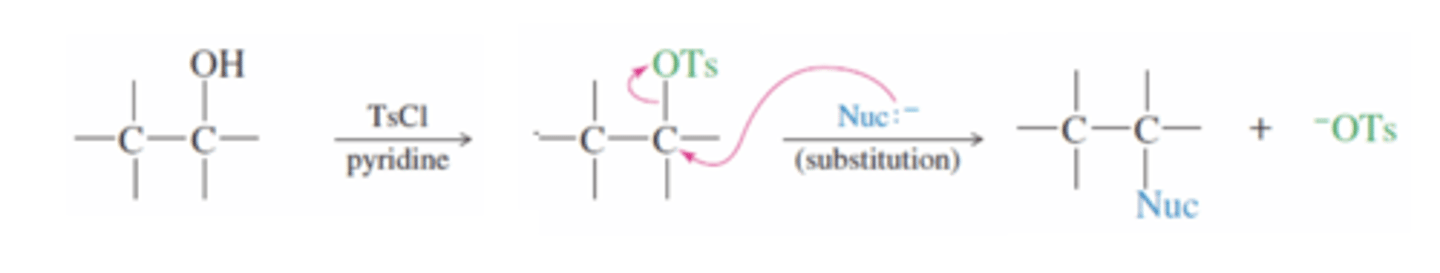
Tosylate
-A great leaving group because the tosylate ion is stabilized by resonance
-Alcohol can react with TsCl in the presence of pyridine to add in nucleophilic substitution
→Ts bonds to the O in the alcohol and displaces the H then since Tosylate is a great leaving group, a Nucleophile can attack the carbon from the back and the OTs will leave
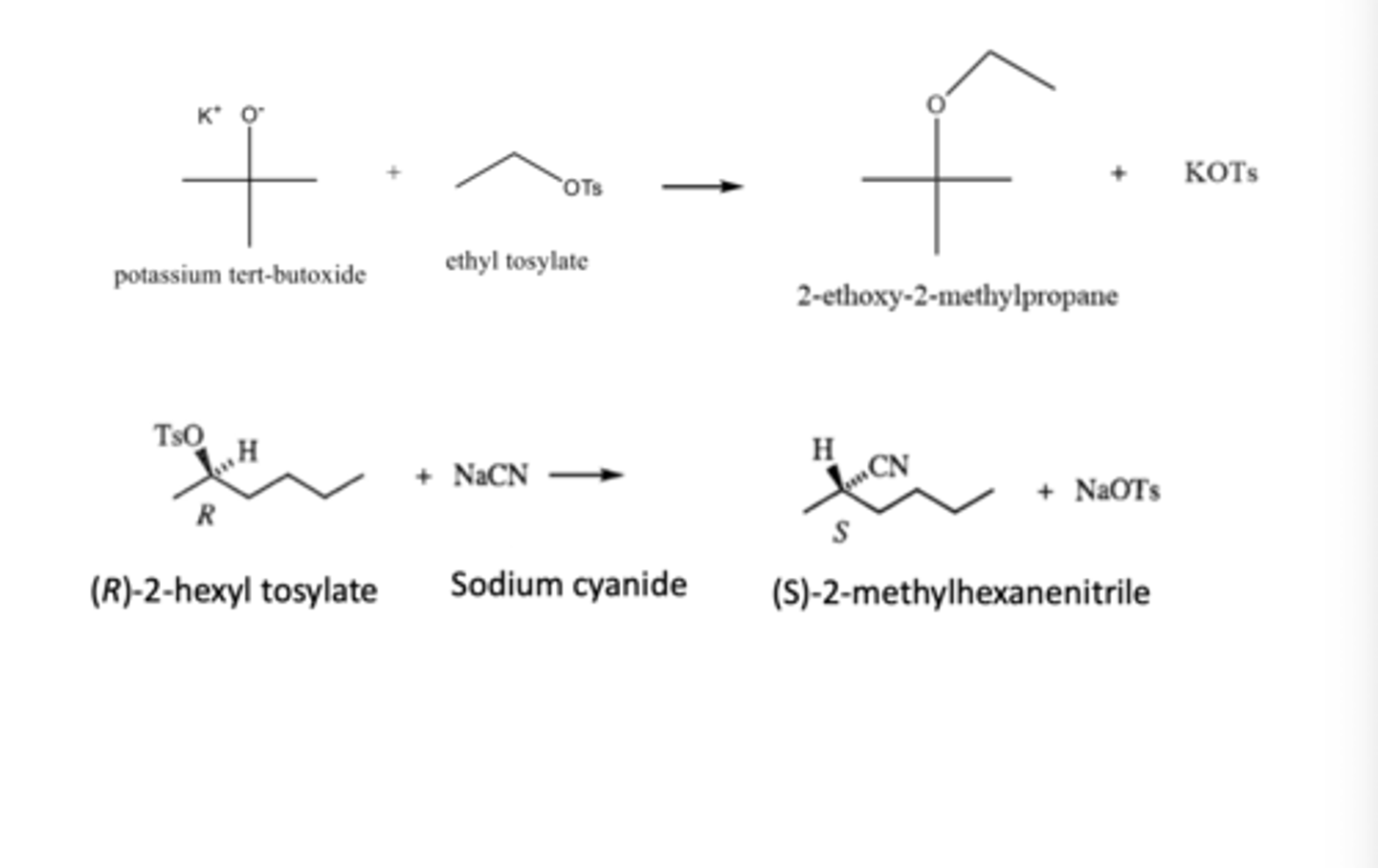
Predict the major products:
a) Potassium ter-butoxide + ethyl tosylate
b) (R)-2-hexyl tosylate + sodium cyanide
Alcohol to Alkane
-Reduction
1) Dehydrate with conc. H2SO4 (produces an alkene) and then Add H2 using Pt Catalyst
→ Protonation to alkene and then addition of hydrogens to break double bond
2) React alcohol with TsCl and Reduce with LiAlH4
→ Make OH a leaving group and then add Hydride
3° and 2° alcohols + HBr
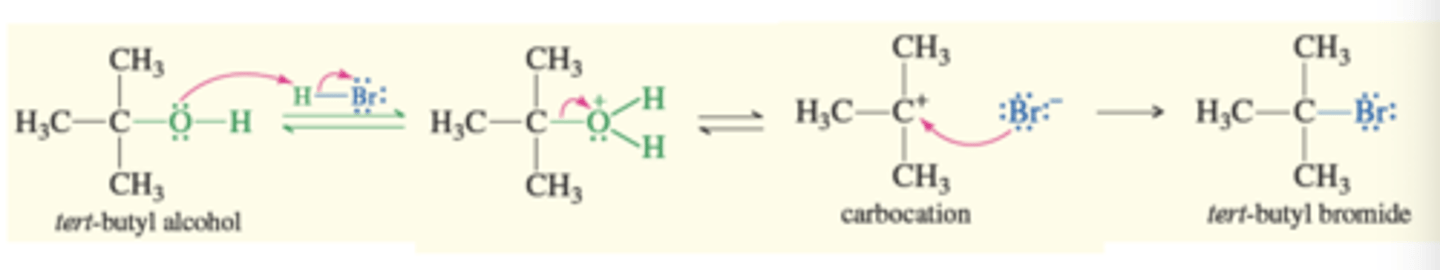
Alcohol to Alkyl Halide
SN1
-Used for 3° and 2° alcohols
→ Protonation converts the hydroxy group into a good leaving group, water leaves forming carbocation and bromide ion attacks carbocation
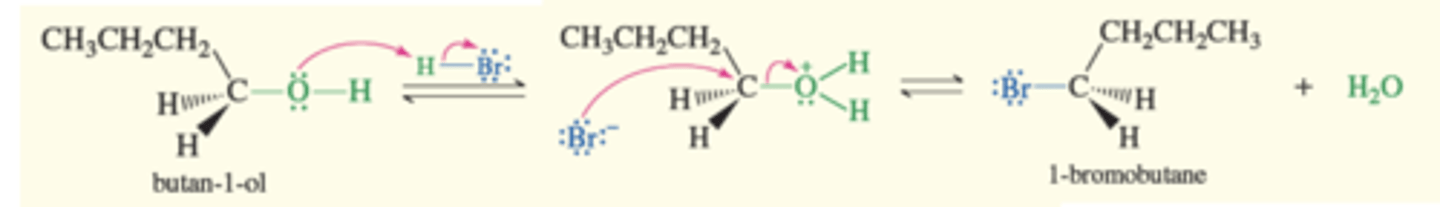
1° alcohols + HBr
Alcohol to alkyl halide
→ Protonation converts hydroxy group into a good leaving group and bromide displaces the water to give the alkylbromide
3° and 2° alcohols + HCl in the presence of ZnCl2 or Lucas Reagents
Alcohol to alkyl halide
SN1
-Chloride is a weaker nucleophile than bromide and needs ZnCl2 which is stronger than a proton
→ZnCl2 binds to oxygen in alcohol to create a good leaving group, when it leaves with the -OH it produces a stable carbocation that the chlorine ion attacks
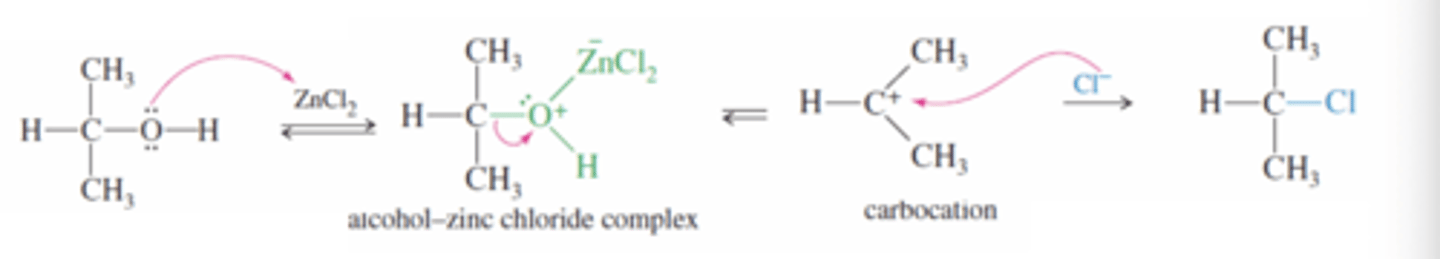
1° Alcohols + HCl
Alcohol to alkyl halide
SN2
→ZnCl2 creates a leaving group and at the same time it leaves the chlorine ion attacks from the back
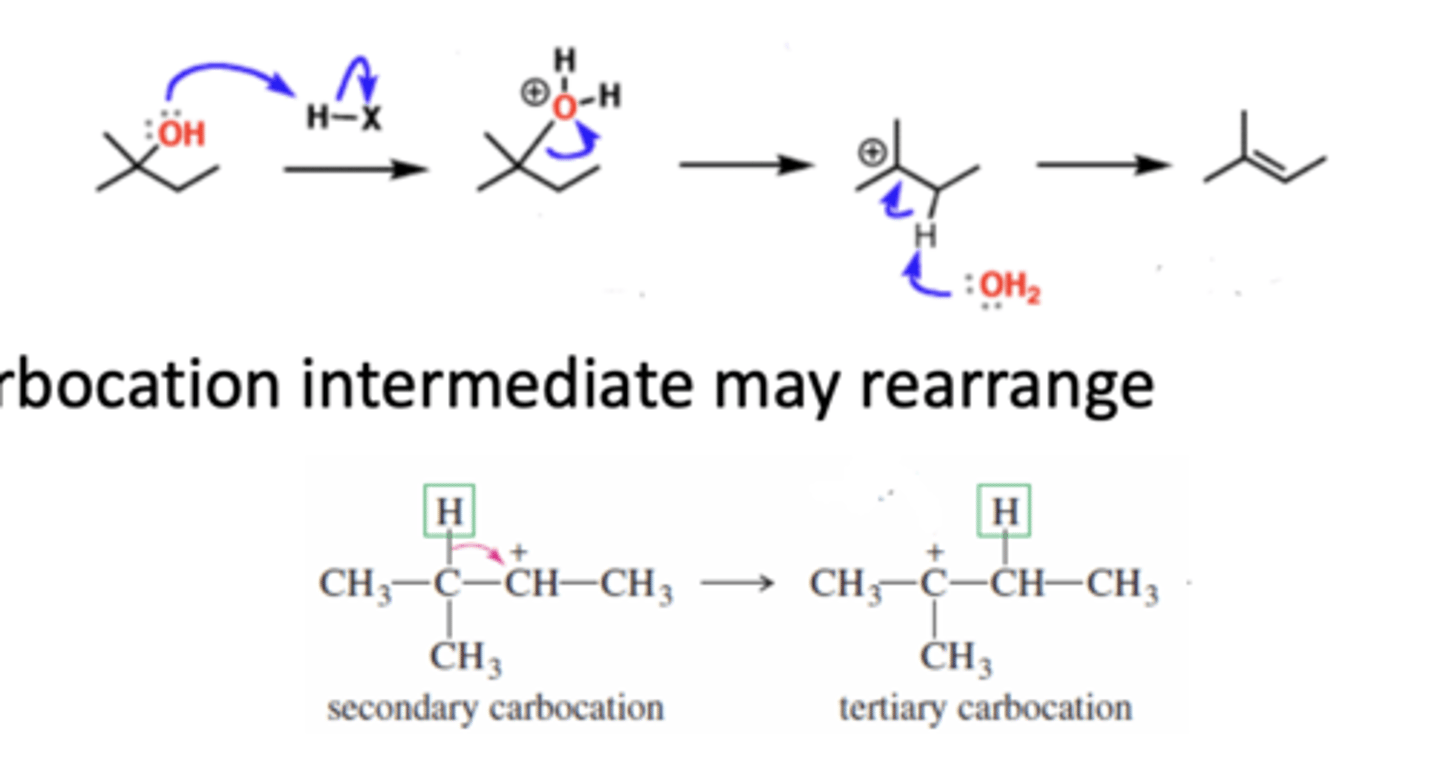
Limitations of HX Reactions
1) HI does not react with alcohol
2) 1° and 2° alcohols react very slowly
3) Elimination reactions will produce alkenes instead of alkyl halides because of water acting as a base and deprotonating
4) Carbocation intermediate may rearrange to become most stable
Alcohol + PBr3
Alcohol to Alkyl Bromide
-PBr3 is a strong electrophile
-Alcohol displaces bromide ion from PBr3 to create a bond
-Bromide ion does SN2 attack on alkyl group displacing the hydroxy-PBr2 group and forming alkyl bromide
Alcohol + (PCl3/PCl5)
Alcohol to alkyl chloride
Chlorine from phosphorus molecule attacks alkyl group at the same time oxygen in alcohol attacks phosphorus to create a cleaving group
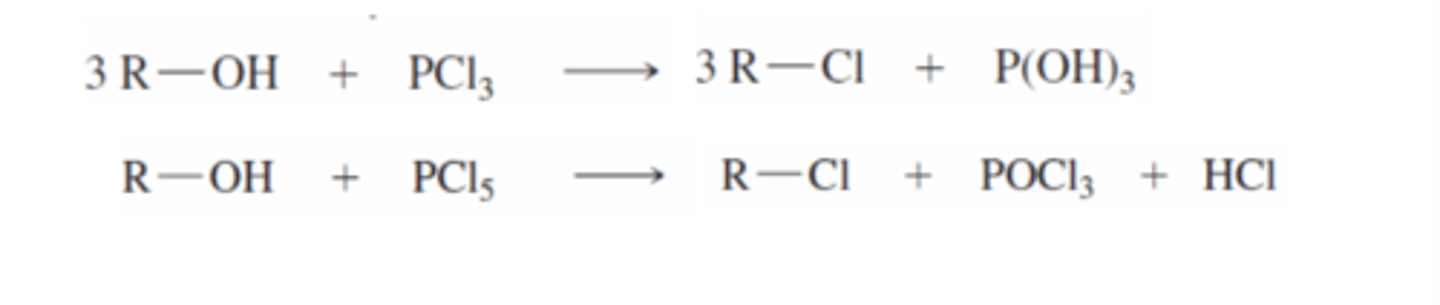
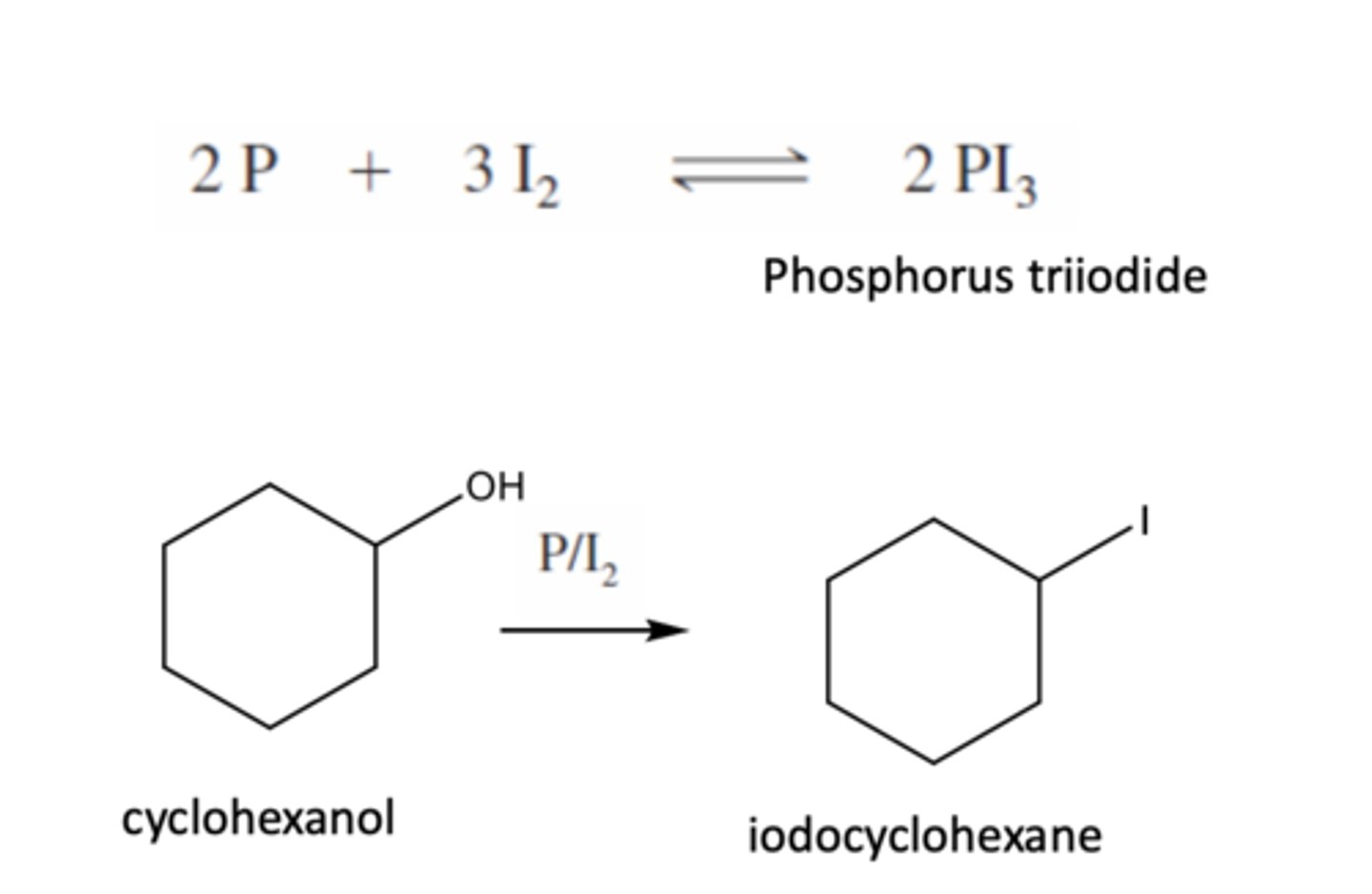
Alcohol + PI3
To convert alcohol to alkyl iodide
→PI3 is not stable to be stored
→Generated in reaction by the reaction of phosphorus with iodine
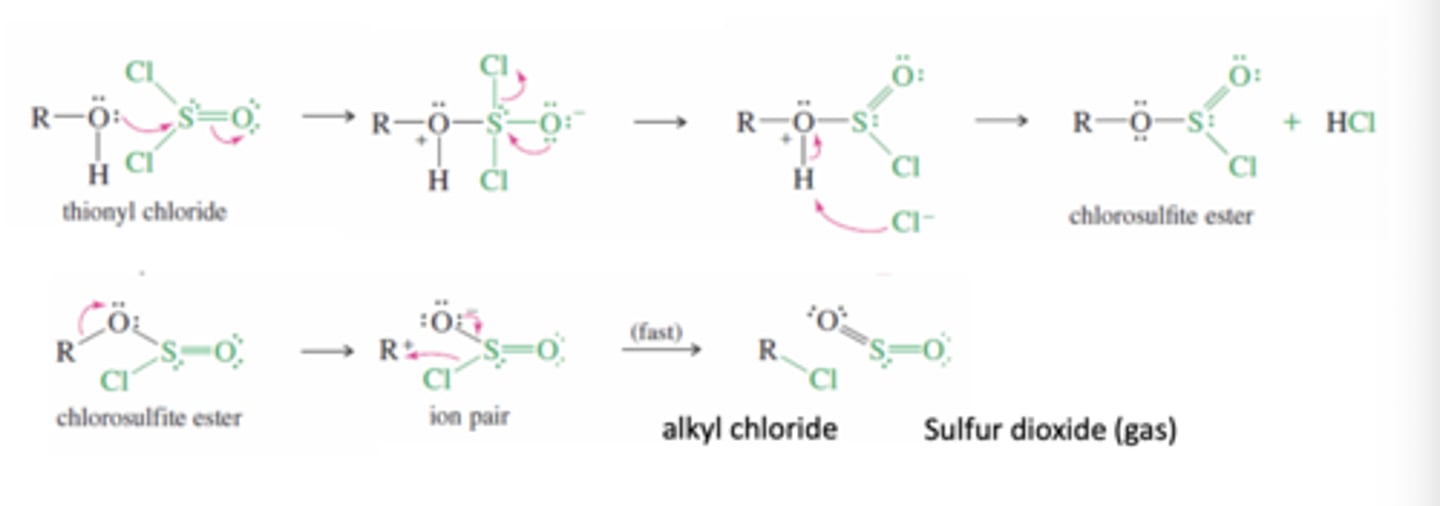
Alcohol + SOCl2 (Thionyl Chloride)
To convert Alcohol to Alkyl Chlorides
-Oxygen attacks the electrophilic sulfur atom of SOCl2
-Chloride ion is expelled
-Intermediate is deprotonated to give the chlorosulfite ester which ionizes and produces a carbocation
-chloride quickly attacks carbocation
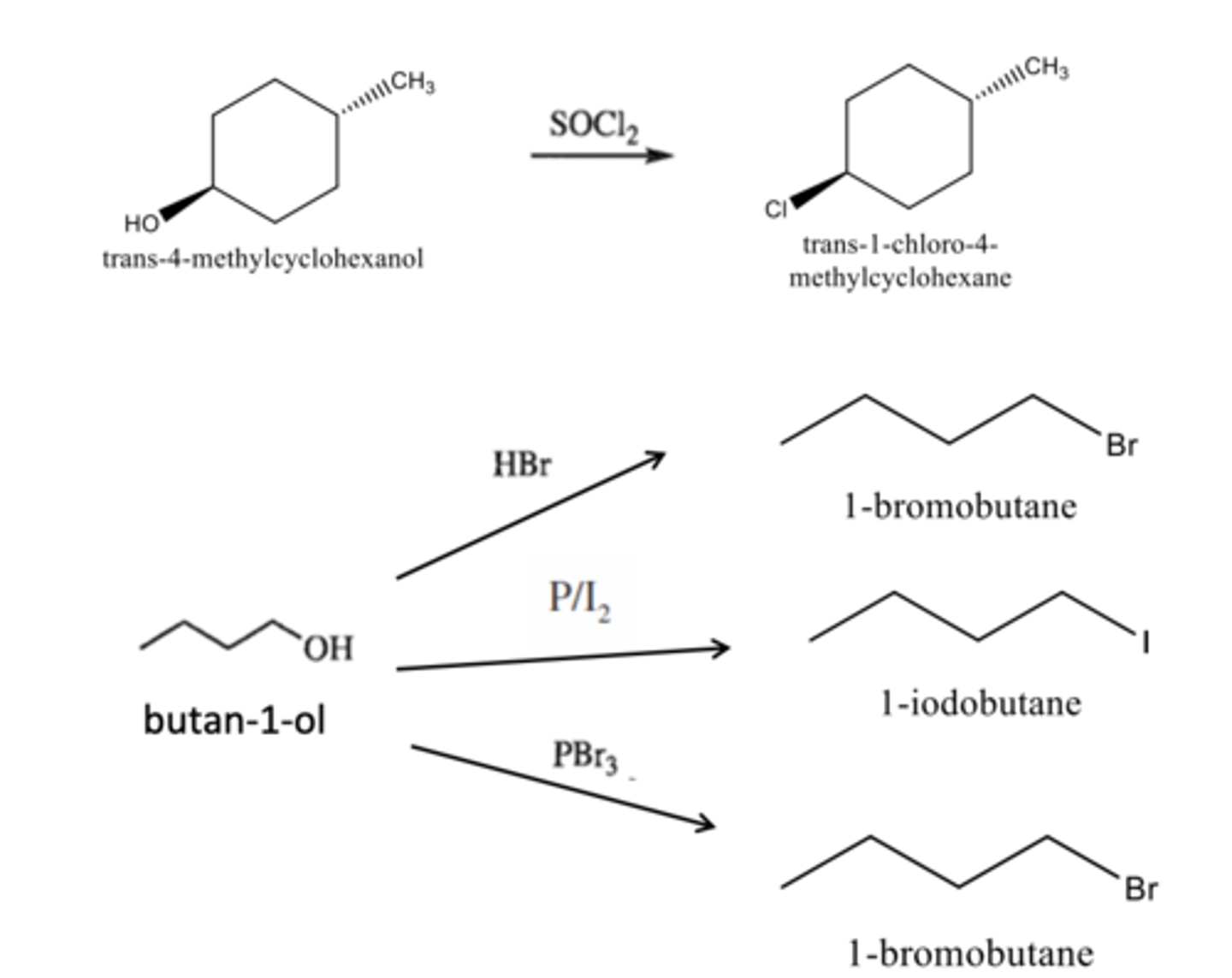
a) Trans-4-methylcyclohexanol + SOCl2
b) butan-1-ol + HBr
c) butan-1-ol + P/I2
d) butan-1-ol + PBr3
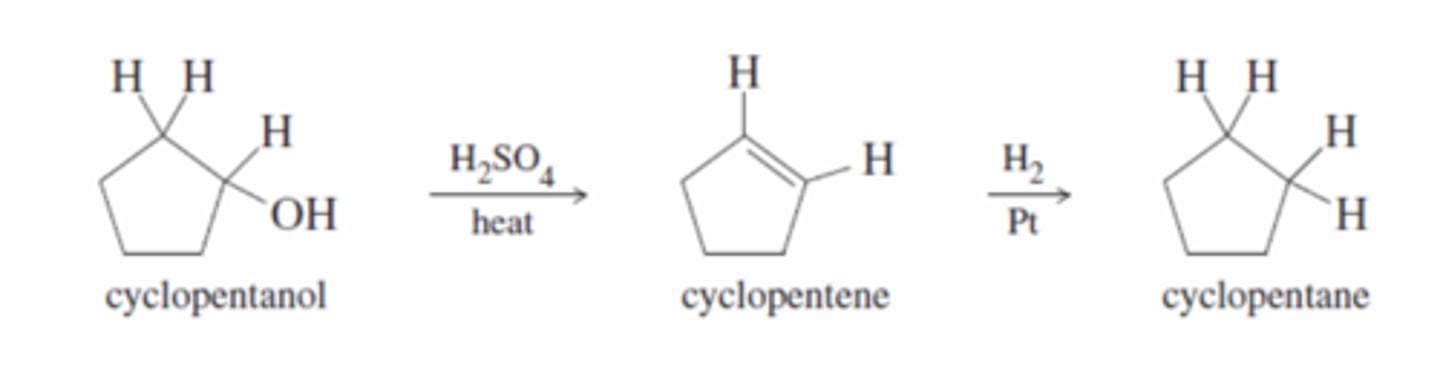
Acid-Catalyzed Dehydration of an Alcohol
H2SO4
-Protonation converts the hydroxy group to a good leaving group
-Water leaves forming a good carbo cation
-Water removes a proton to produce the alkene (most substituted)
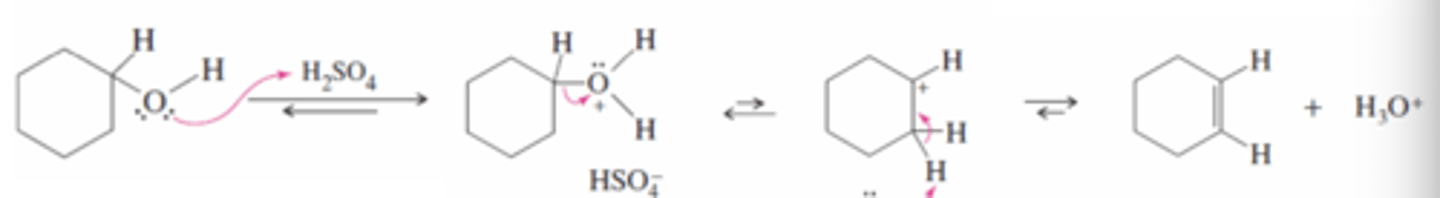
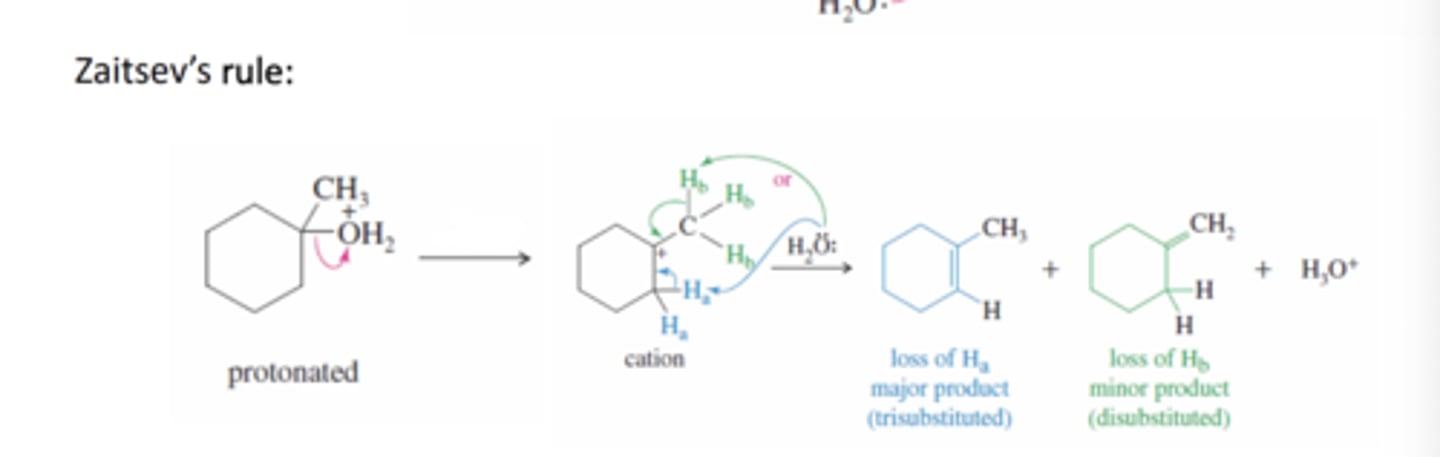
Zaitsev's Rule
The most substituted alkene is formed preferentially by deprotonating the least substituted carbon adjacent to the carbocation
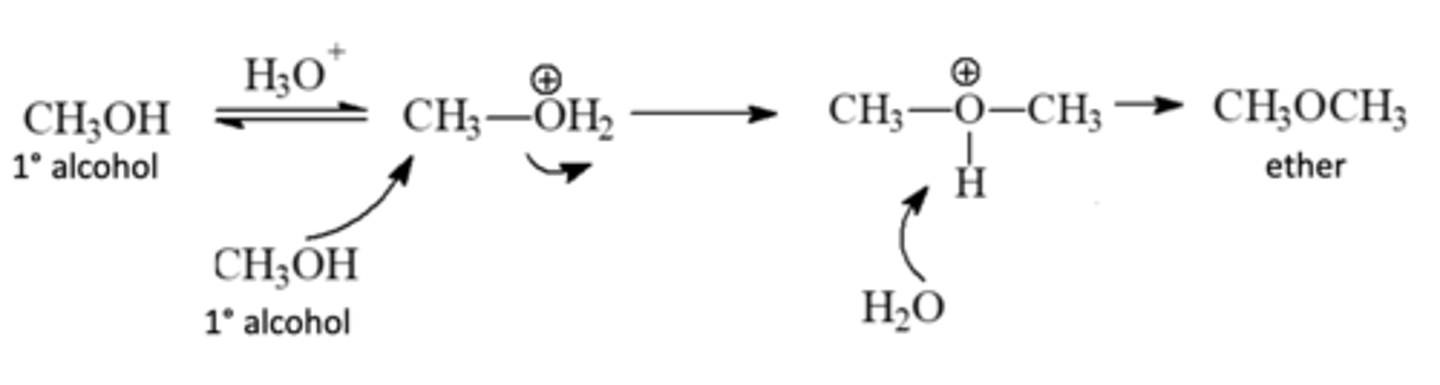
Bimolecular Condensation
Forms Ethers
-Protonated 1° alcohols can be attacked by another molecule of the alcohol and undergo an SN2 displacement
(Two alcohols combining and then being deprotonated)
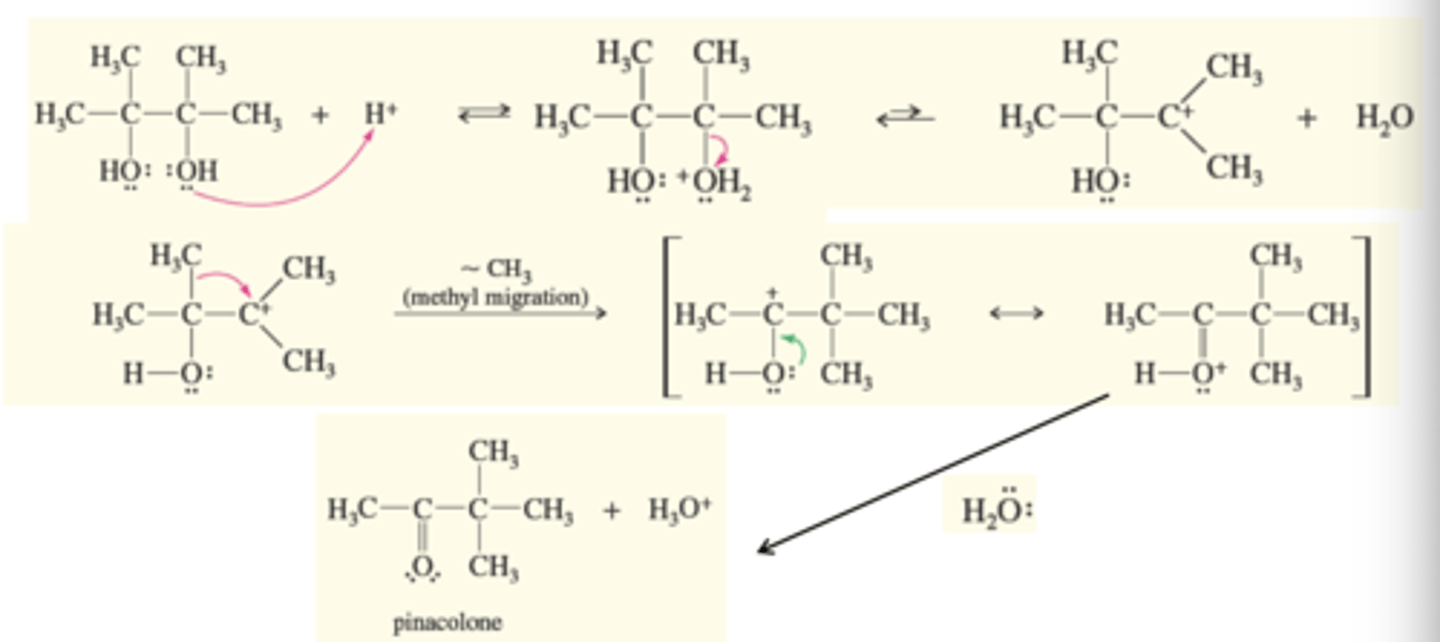
Pinacol Rearrangement
Unique reaction of diols
-One of the hydroxy oxygens get protonated
-A water molecule leaves forming a carbocation
-A methyl migrates to form a resonance-stabilized carbocation
-Deprotonation give s the pinacolone product
Esterification
Addition of an alcohol to an acid
Mechanism:
1) protonation of acid O
2) Electron movement creates a carbocation where alcohol can attack
3) Alcohol attached to acid is deprotonized and acid oxygen is protonized
4) water leaves
5) Resonance stabilization with O + to make acid O positive
6) Acid Oxygen is deprotonated
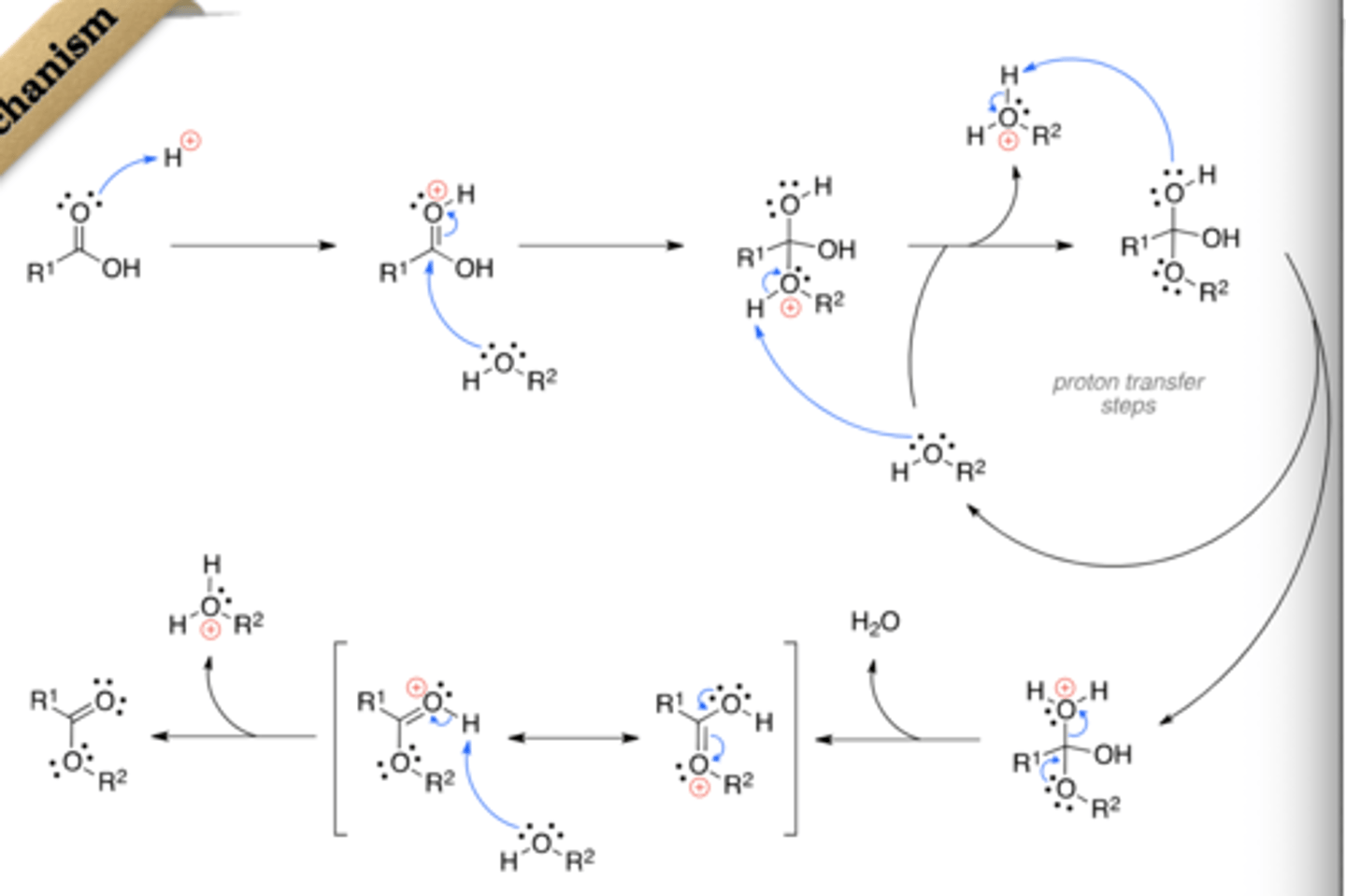
Fischer Esterification
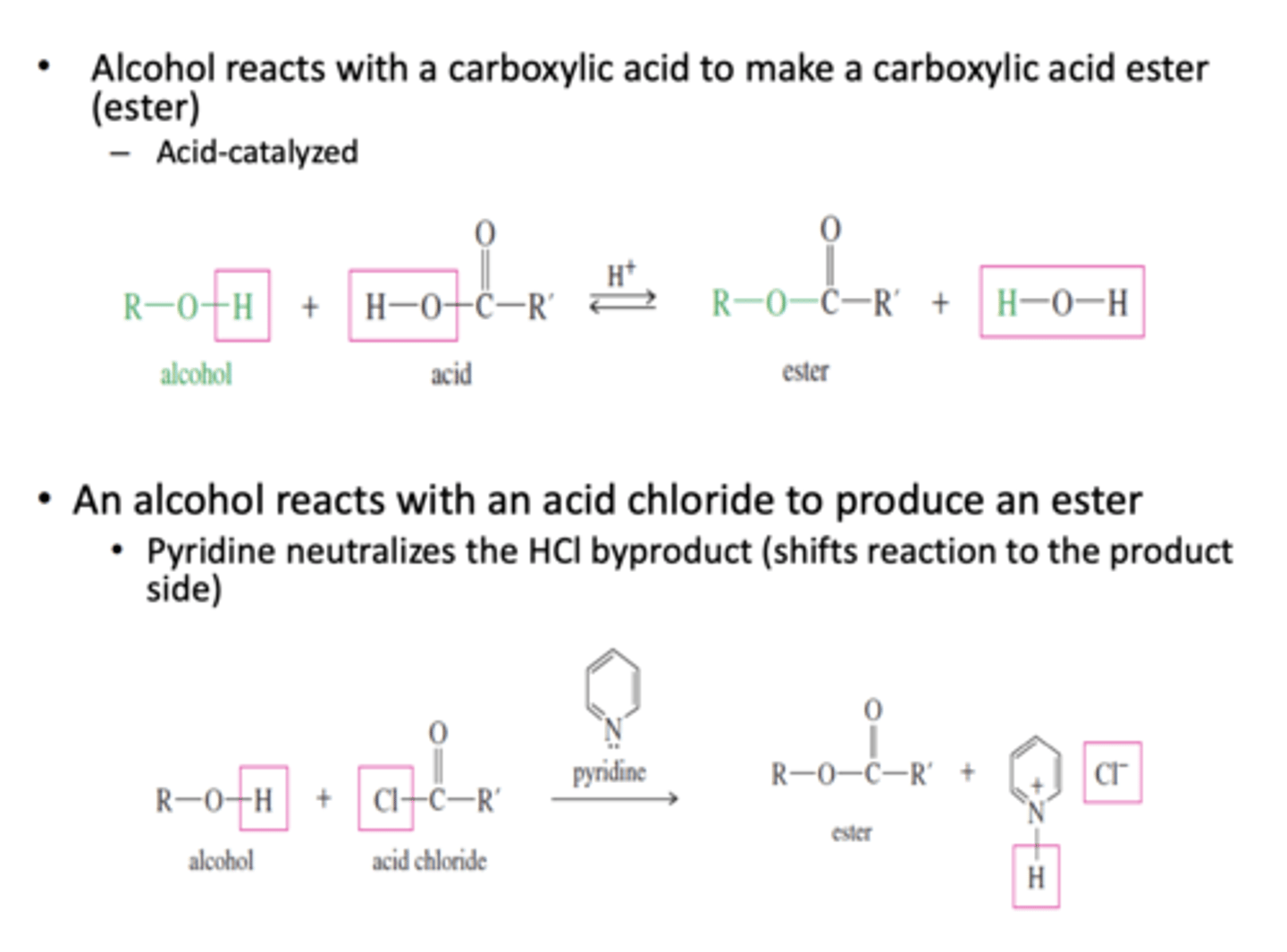
1) Alcohol reacts with a carboxylic acid to make a carboxylic acid ester (acid-catalyzed)
2) Alcohol reacts with an acid chloride to produce an ester in the presence of pyridine which neutralizes the HCl byproduct
Tosylate Esterification
1) Alcohol reacts with p-Toluenesulfonic acid (TsOH)
2) Alcohol reacts with para-toluenesulfonyl chloride (TsCl)
→reaction is pyridine catalyzed
→ H and Cl become a molecule with pyridine
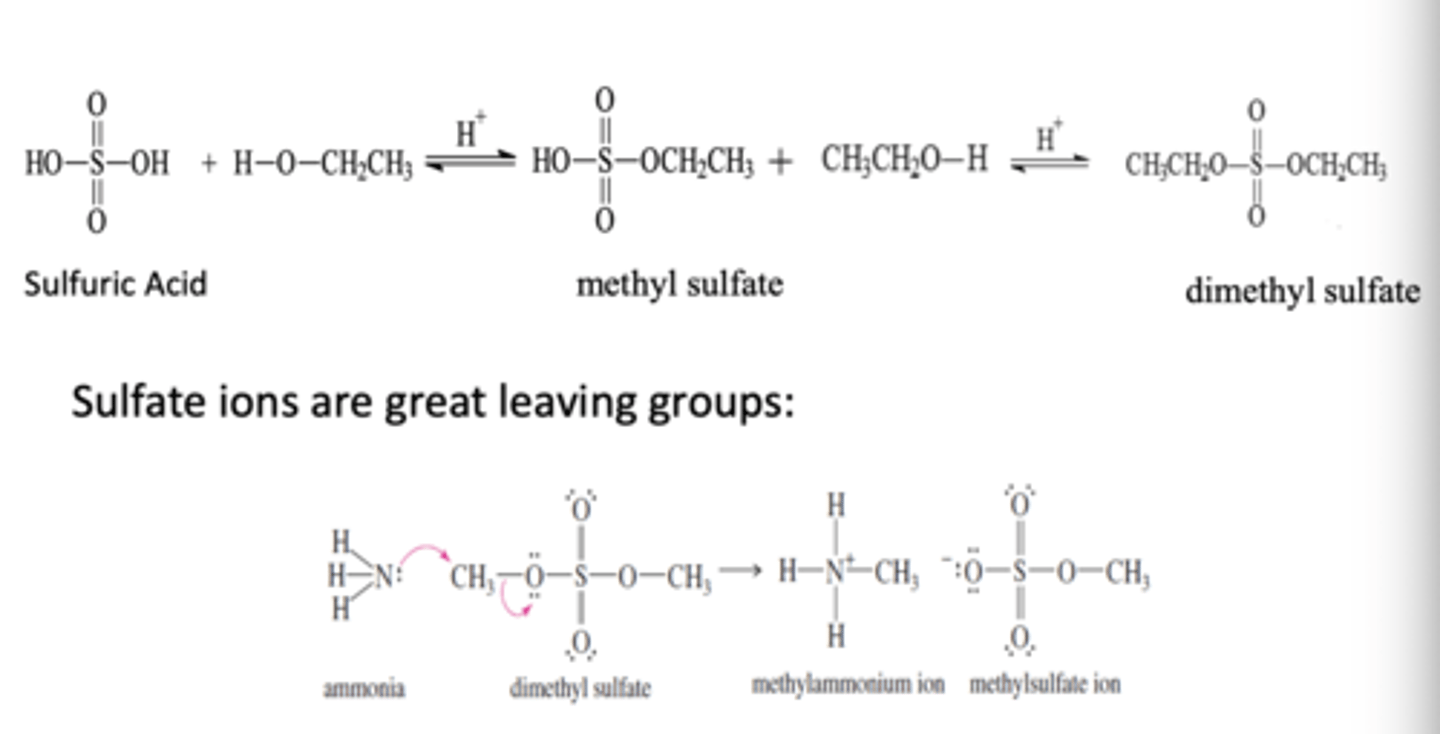
Sulfate Esterification
Alcohol + sulfuric acid
- sulfur atom in the acid is not bound to an alkyl group
-sulfate ester can react with another alcohol molecule
-sulfate ions are great leaving groups
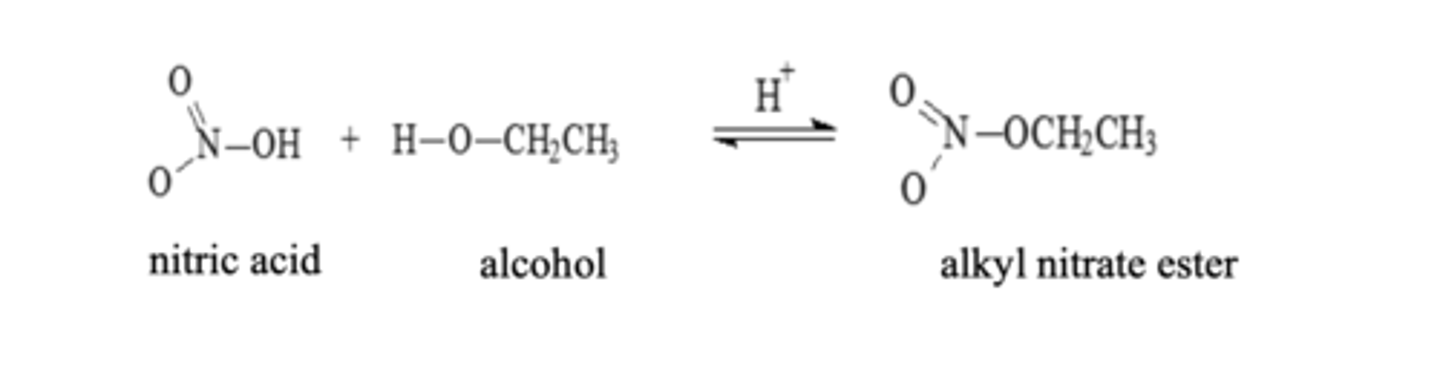
Nitrate Esterification
Alcohol + Nitric Acid
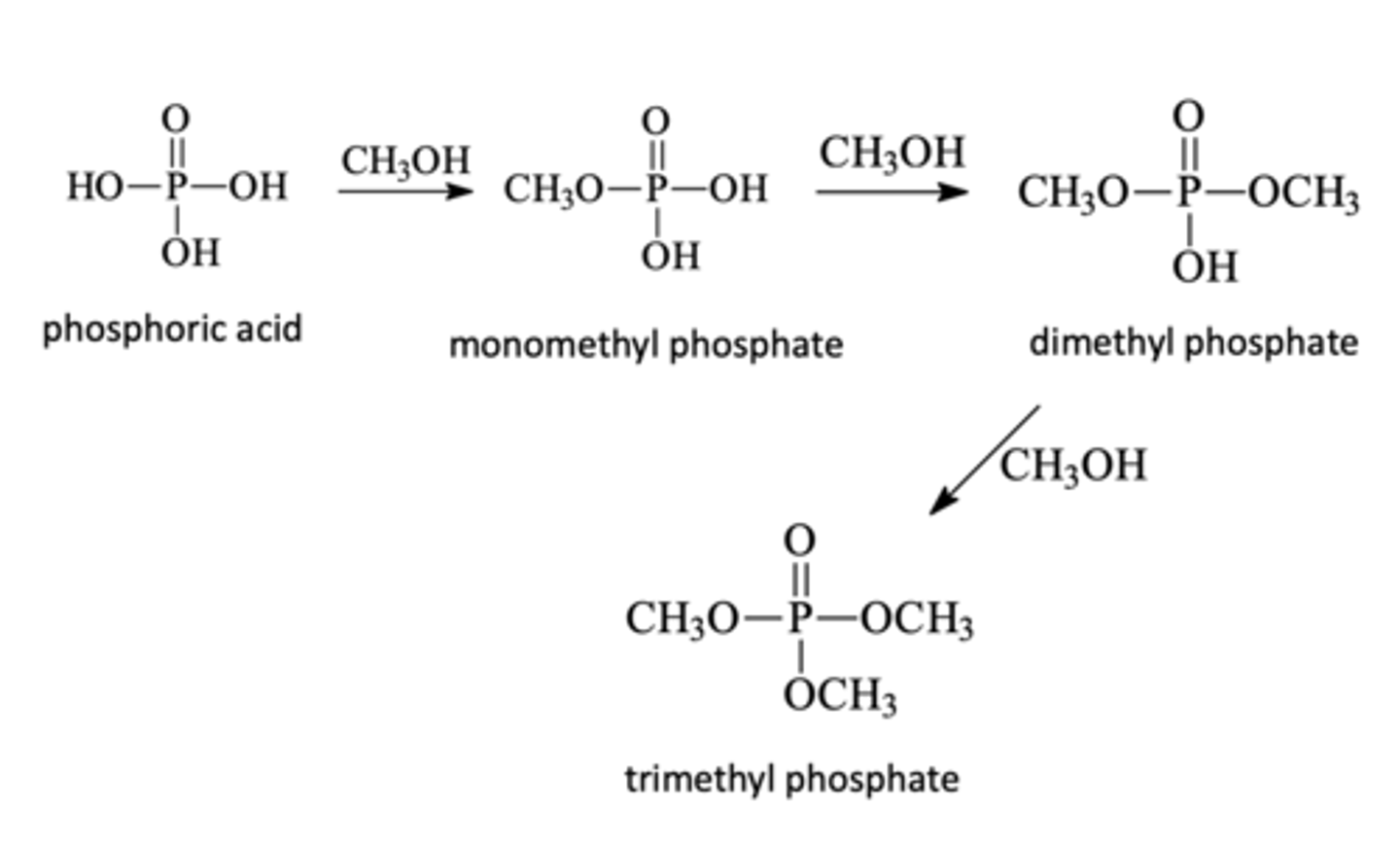
Phosphate Esterification
Alcohol + Phosphoric Acid
→ The central phosphorus can bing three alkoxy groups
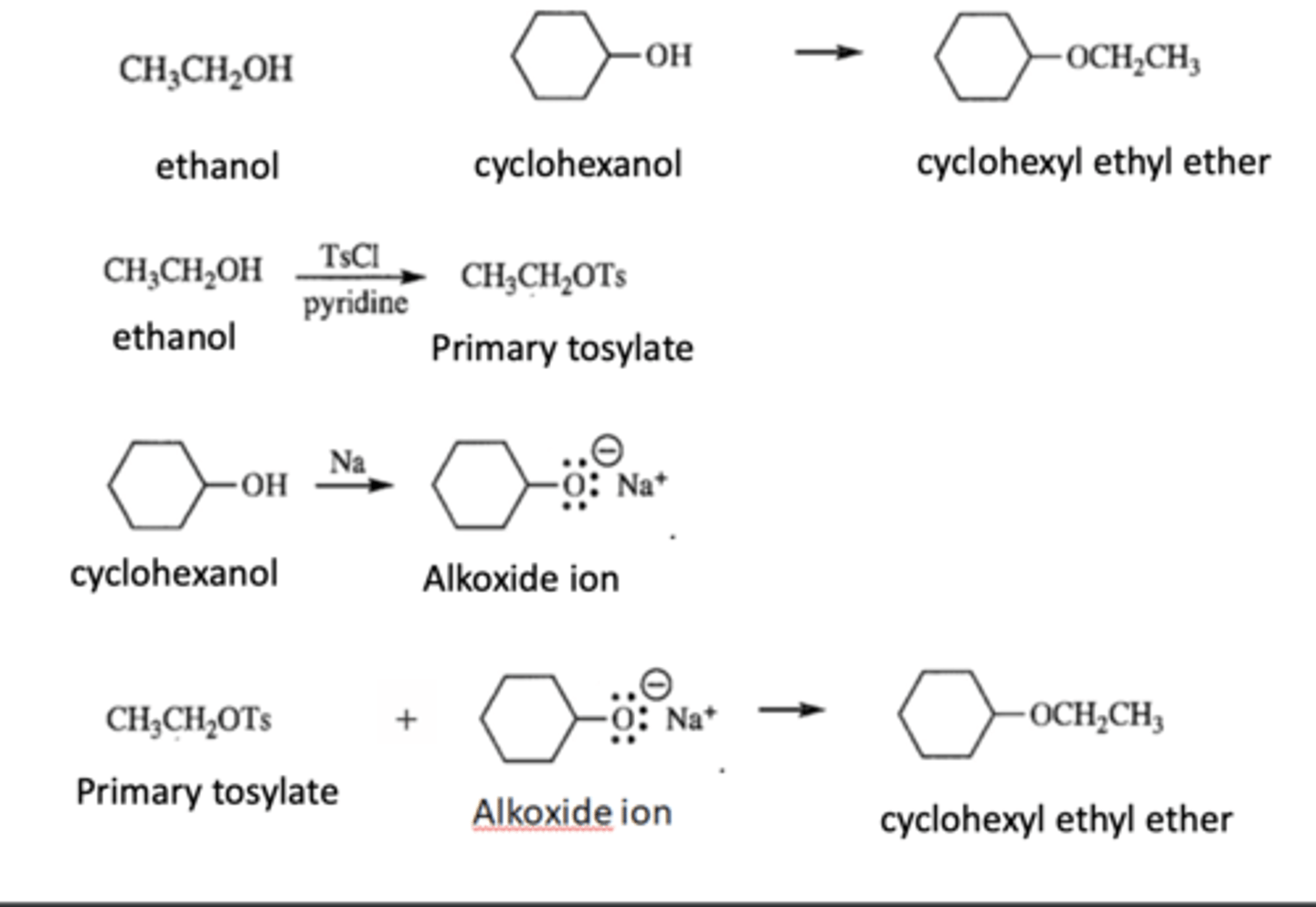
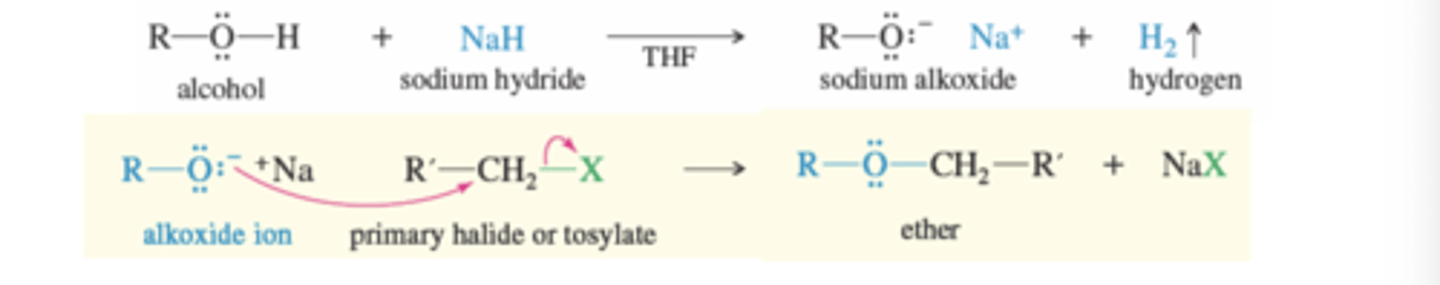
Williamson Ether Synthesis
An Alkoxide ion reacts with 1° alkyl halides or tosylates to produce an ether
Mechanism:
1) Alkoxide ion is formed using alcohol + NaH in the presence of THF
→Hydride attacks alcohol H to create gas
2) Alkoxide ion attacks the carbon and displaces the leaving group in an SN2 mechanism
→ One step with a highly unstable transition state (Bonds break and form at the same time)
How can Ethanol and Cyclohexanol be used to synthesize cyclohexyl ethyl ether
Williamson Ether Synthesis
-Need to form a primary tosylate and an alkoxide ion