Chemistry - Ch 18 - Aqueous Ionic Equilibrium
1/34
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
buffer
a solution containing significant amounts of both a weak acid and its conjugate base (or a weak base and its conjugate acid)
resists pH change by neutralizing added acid or added base
(acid neutralizes added base or base neutralizes added acid)
e.g. HC2H3O2 (acetic acid) and NaC2H3O2 (sodium acetate; conjugate base)
common ion effect
tendency for a common ion to decrease the solubility of an ionic compound or to decrease the ionization of a weak acid or weak base
Henderson-Hasselbalch equation
used to calculate pH of a buffer solution
relates the pH of a buffer solution to the initial concentration of the buffer components
(simplifies the calculation of a buffer solution)
can be used as long as the x is small approximation is valid (because equilibrium concentrations of HA and A- will be essentially identical to initial concentrations, thus [H3O+]=Ka)
note: x is small approximation is valid because so little of the weak acid ionizes compared to its initial concentration; [H3O+]=Ka as long as weak acid and conjugate base concentrations are equal in the buffer
![<p>used to calculate pH of a buffer solution</p><p>relates the pH of a buffer solution to the initial concentration of the buffer components</p><p>(simplifies the calculation of a buffer solution)</p><p>can be used as long as the x is small approximation is valid (because equilibrium concentrations of HA and A- will be essentially identical to initial concentrations, thus [H3O+]=Ka)</p><p>note: x is small approximation is valid because so little of the weak acid ionizes compared to its initial concentration; [H3O+]=Ka as long as weak acid and conjugate base concentrations are equal in the buffer</p>](https://knowt-user-attachments.s3.amazonaws.com/e1f8c9e9-4d49-4c31-b6df-dcfa9b00cc5d.jpeg)
approximate pH of a buffer solution (without calculation)
if [acid]=[base]: pH=pKa
if [acid]>[base]: pH<pKa
if [acid]<[base]: pH>pKa
pH = pKa
if concentration of acid equals concentration of conjugate base
pH > pKa
if concentration of acid is less than concentration of conjugate base
pH < pKa
if concentration of acid is greater than concentration of conjugate base
2 parts of calculating change in pH
stoichiometry calculation
equilibrium calculation
stoichiometry calculation
first part of calculating pH change when an acid or base is added to a buffer
calculation of how the addition changes the relative amounts of acid and conjugate base
if strong acid is added to buffer and neutralized, an amount of the weak base is converted into its conjugate acid
if strong base is added to buffer and neutralized, an amount of the weak acid is converted to its conjugate base
equilibrium calculation
calculation of pH based on the new amounts of acid and conjugate base after the stoichiometry calculation
use ICE table based on a balanced equation for the ionization of the acid
most effective buffer
most resistant to pH changes
when concentrations of acid and conjugate base are equal
buffers become less effective as the difference in relative amounts of acid and conjugate base increases
effective buffer must have a [base]/[acid] ratio in the range of 0.1 to 10
effective range for a buffering system is pKa±1
buffer capacity
the amount of acid or base that can be added to a buffer without causing a large change in pH
increases with increasing absolute concentrations of the buffer components
overall buffer capacity increases as the relative concentrations of the buffer components become more similar (ratio of buffer components gets closer to 1)
acid-base titration
procedure in which a basic (or acidic) solution of unknown concentration reacts with an acidic (or basic) solution of known concentration in order to determine the concentration of the unknown
pH is monitored with either a pH meter or indicator
as acid and base combine, they neutralize each other
indicator
a substance whose color depends on the pH
equivalence point
the point in titration when the number of moles of base is stoichiometrically equal to the number of moles of acid
when titration is complete
neither reactant is in excess, the number of moles of the reactants are related by the reaction stoichiometry
titration curve (or pH curve)
a plot of the pH of the solution during a titration
titration of a strong acid with a strong base
solution starts with containing only strong acid (e.g. HCl)
NaOH is added
initial pH is pH of the strong acid solution
before equivalence point, calculate [H3O+] by subtracting the number of moles of added OH- from the initial moles of H3O+ and dividing by the total volume
pH will always be 7.0 at equivalence point (at 25*C)
beyond equivalence point, calculate [OH-] by subtracting the initial number of moles of H3O+ from the moles added of OH- and dividing by the total volume
![<p>solution starts with containing only strong acid (e.g. HCl)</p><p>NaOH is added</p><p>initial pH is pH of the strong acid solution</p><p>before equivalence point, calculate [H3O+] by subtracting the number of moles of added OH- from the initial moles of H3O+ and dividing by the total volume</p><p>pH will always be 7.0 at equivalence point (at 25*C)</p><p>beyond equivalence point, calculate [OH-] by subtracting the initial number of moles of H3O+ from the moles added of OH- and dividing by the total volume</p>](https://knowt-user-attachments.s3.amazonaws.com/682779b9-25de-455f-9aa5-d449803b48eb.png)
titration of a strong base with a strong acid
solution starts with strong base
acid is added, pH decreases
pH at equivalence point is 7.0
(similar to titration of strong acid with strong base, but flipped)
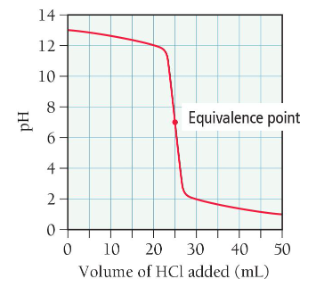
titration of a weak acid with a strong base
begin with weak acid, add strong base until equivalence point is reached
find initial moles of acid
determine number of moles of NaOH needed to neutralize acid
find volume of the base using molarity
solve using Ka and an ICE table
solve for equivalence point
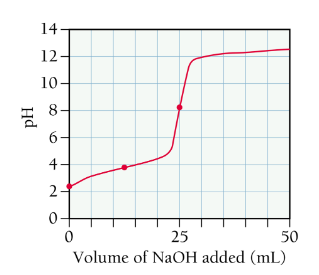
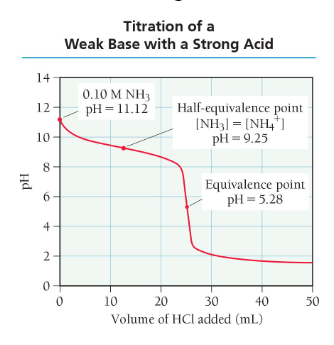
titration of a weak base with a strong acid
similar to calculating points along the curve for the titration of a weak acid with a strong base
solution starts basic and has an acidic equivalence point
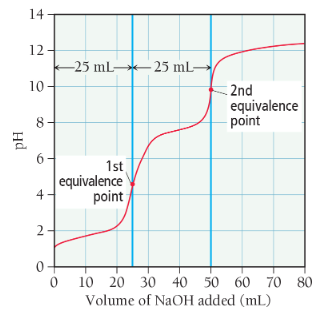
titration of a polyprotic acid
pH curve has multiple equivalence points (e.g. titration of a diprotic acid with strong base will result in a pH curve with 2 equivalence points)
end point
the point in titration where an indicator changes color
used to determine the equivalence point
solubility product constant
the equilibrium constant for a chemical equation representing the dissolution of an ionic compound
usually = products multiplied together and raised to the power of coefficients (since solids are omitted and usually reactant is a solid)
can be used to calculate molar solubility of a compound
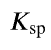
effect of a common ion on solubility
when a compound with a common ion is added to another solution, solubility either decreases or increases
the solubility of an ionic compound is lower in a solution containing a common ion than in pure water2
effect of pH on solubility
in general, the solubility of an ionic with a basic anion increases with increasing acidity (decreasing pH)
unsaturated solution
a solution that contains less than the equilibrium amount of dissolved ions (when all solid has already been dissolved)
reaction proceeds to the right
Q < Ksp
saturated solution
a solution whose reaction is at equilibrium
Q = Ksp
supersaturated solution
a solution that contains more solute than solvent
forms a precipitate when sufficiently disturbed
Q > Ksp
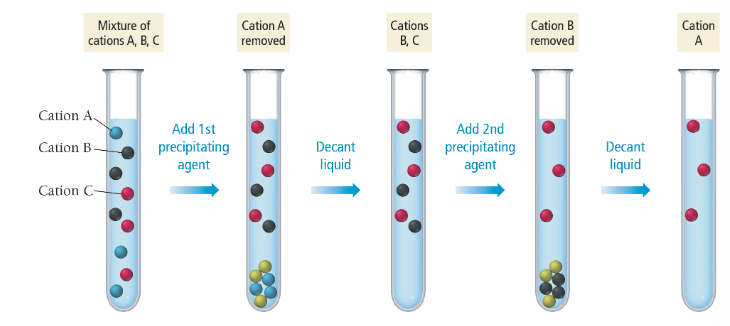
selective precipitation
a process involving the addition of a reagent to a solution that forms a precipitate with one of the dissolved ions but not the others
requires a difference in Ksp by a factor of at least 10³
qualitative analysis
a systematic way to determine the ions present in an unknown solution
selective precipitation can be used for it
in the past, used to determine the metals present in a sample
quantitative analysis
a systematic way to determine the amounts of substances in a solution or mixture
complex ion
an ion containing a central metal ion bound to one or more ligands
e.g. Ag(H2O)2+
ligand
a neutral molecule or ion that acts as a Lewis base with the central metal ion (reminder: Lewis acids donate an electron pair)
e.g. H2O in Ag(H2O)2+
will be replaced if a stronger Lewis base is put into a solution containing the subject ion (e.g. NH3)
formation constant
the equilibrium constant associated with the reaction for the formation of a complex ion
calculated the same as any other K value
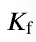
effect of complex ion equilibria on solubility
the solubility of an ionic compound containing a metal ion that forms complex ions increases in the presence of Lewis bases that complex with the cation