Reactivity Series
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
What is the reactivity series, in order of decreasing reactivity, ?
Potassium
Sodium
Lithium
Calcium
Magnesium
Aluminium
Carbon
Since
Iron
Hydrogen
Copper
Silver
Gold
metal + water --->
metal hydroxide + hydrogen
metal + steam --->
metal oxide + hydrogen
What happens when we react metals in the reactivity series with water ?
( which metals do what )
metals above hydrogen :
react with water or steam to produce, metal hydroxide + hydrogen or metal oxide + hydrogen
and effervescence is produced
Metals below hydrogen don't react with water/steam
What happens when we react metals in the reactivity series with dilute acid ?
potassium, sodium, lithium and calcium aren't reacted with dilute acids, because it is too reactive
( apart from very diluted calcium)
metals below this but above hydrogen , react and produce effervescence:
magnesium- vigorously
aluminium- vigorous but slow to react
zinc and iron- slow to react not very vigorous
metals below hydrogen don't react with dilute acid
metal + dilute sulfuric acid -->
metal sulfate + hydrogen
metal + dilute hydrochloric acid --->
metal chloride + hydrogen
metal + acid -->
salt + hydrogen
What conditions are required for iron to rust ?
iron rusts in the presence of oxygen and water
Fe2O3 . XH2O
What are the methods of preventing rusting ?
Barriers
Galvanising
Sacrificial Protection
How does the barrier method prevent rusting ?
it coats the iron in a paint, oil, grease or plastic or a metal below it in the reactivity series
How does galvanising prevent rusting ?
it coats iron in a layer of zinc, as zinc is more reactive than iron and reacts more with oxygen and water
and prevents water and oxygen reaching the iron
It is both barrier and sacrificial protection
How does sacrificial protection prevent rusting ?
Sacrificial protection is adding metal blocks which are more reactive than iron, to prevent the iron from reacting with oxygen and water in the atmosphere
What does oxidation mean ?
oxidation is the gain of electrons and the ( gain of oxygen )
What does reduction mean ?
reduction is the loss of oxygen and loss of electroncs
What does the term redox reaction mean ?
a reactions where oxidation and reduction take place simultaneously
What does the term reducing agent mean ?
a substances that reduces something else
(a reducing agent gets gets oxidised in a chemical reaction )
What does the term oxidising agent mean ?
a substance that oxidises something else
( a oxidising agent gets reduced in a chemical reaction )
What is a displacement reaction ?
A reaction in which a more reactive element takes the place of a less reactive element in a compound
How to write a half equation ?
1. Write down the reactant and the product.
2. Balance the atoms.
3. Write the total charge underneath each species in the equation.
4.Balance the charge by adding electrons.
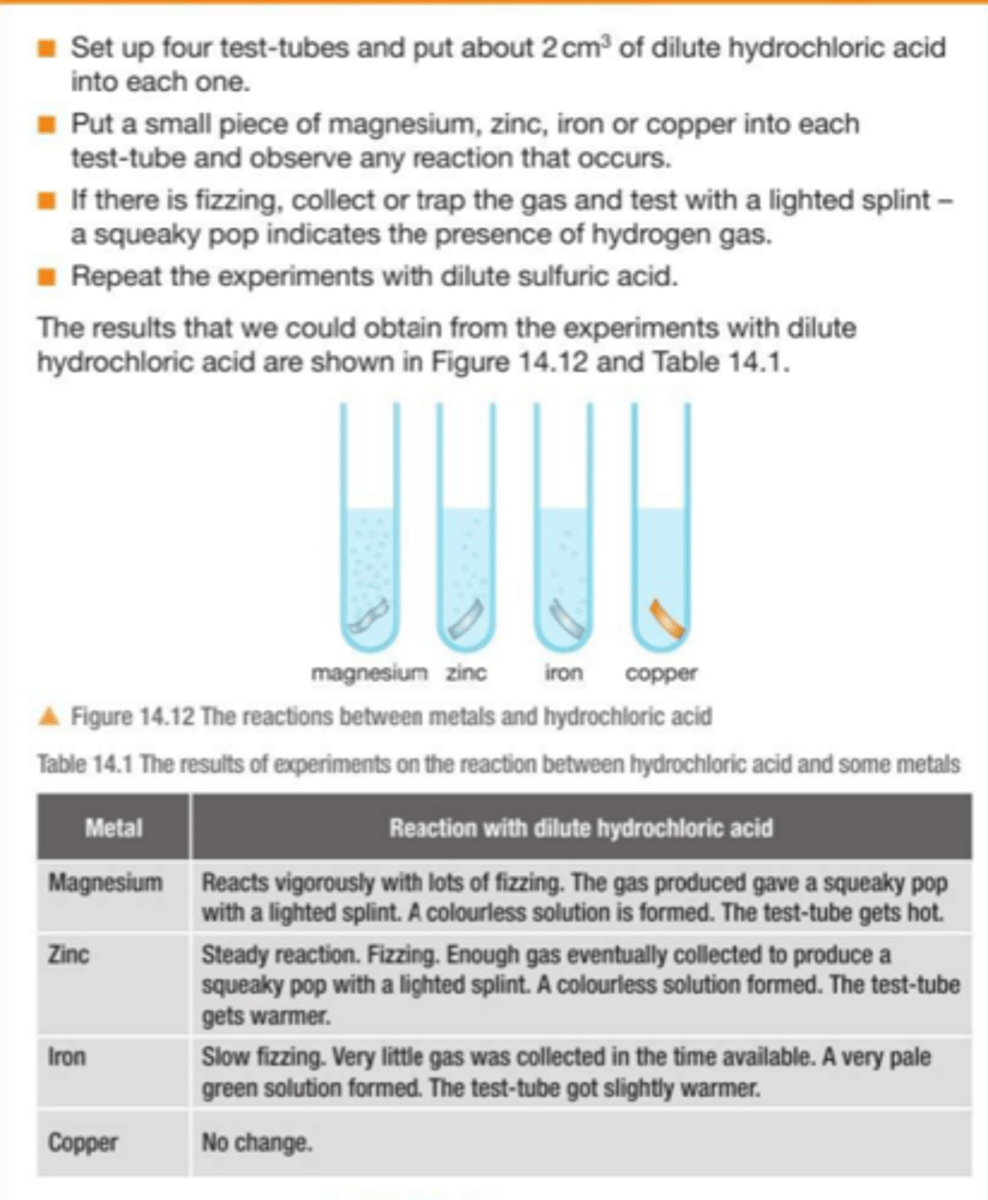
Practical : Investigate reactions between dilute hydrochloric acid and sulfuric acid and metals