The molecular structure of liquids
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
define fluidity
the ability to be moved in a certain direction by small forces
What do fluids not support at rest?
shear stress
What is it called when a molecule oscillates about an equilibrium node for a short period of time, and then jumos to another node?
relaxation time
What does relaxation time depend on?
the liquid’s nature
temperature
How long is the relaxation time?
around 10-13 secs
What is the quasicrystalline structure of liquids?
the crystal-like way the molecules are arranged at the eqm. node but the arrangement is slowly lost as it moves away from the node
Which two properties do ordinary liquids have?
isotropic
amorphous
define isotropic
having a physical property which has the same value when measured in different directions
define amorphous
lacking a clear structure or not crystalline
Give two properties of VdW forces.
repulsive at small distances
attractive at larger distances and decreases away from molecule
What is the radius of action?
the smallest distance between two molecules where the VdW forces become negligible
What is the sphere of molecular action?
a sphere around a molecule with the radius equal to the radius of action of the molecule
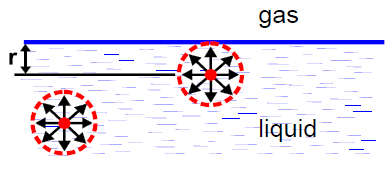
What happens when a force is exerted on a molecule deeper than r?
the molecule is pulled equally in all directions by other liquid molecules so there’s no net force
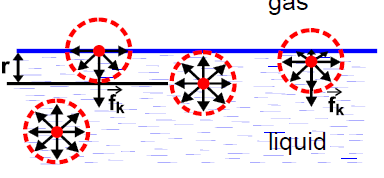
What happens when this force is exerted?
net force fk is normal to the free surface and not zero and directed inwards as less bonds are formed, which is cohesion force
define cohesion pressure (pk)
the sum of the cohesion forces acting on all molecules in the unit area of the surface layer of the liquid
what does cohesion pressure do?
keeps liquids compressed
what is pk for water?
109 Pa
do molecules of the surface layer have higher potential energy or kinetic energy than bulk liquid molecules and why?
they have higher potential energy due to the cohesion pressure
What happens to the energy of the molecule when it moves to the bulk from the surface layer?
the kinetic energy increases
What happens to the energy of the molecule when it moves to the surface layer from the bulk?
the kinetic energy decreases and the potential energy increases
what are surface tension forces?
the forces parallel to the free surface
what shape does the free liquid take?
the shape with minimum potential energy of the surface tension forces, called the spherical shape
define the coefficient of surface tension (alpha)
the sum F of surface tension forces per unit length along an arbitrary line L in the free surface of the liquid
alpha = F/L
what is alpha measured in?
N/m
How can you also define the coefficient of surface tension?
the work needed to increase the area of the surface by one unit of area alpha = A/S
what does alpha depend on?
the liquid’s nature
temperature
concentration and nature of dissolved substances
what are surfactants?
additives that change the alpha of a liquid
what do positive surfactants do and give examples
reduce alpha eg. soap, polymers
what do negative surfactants do and give examples
increase alpha eg. sugars, inorganic salts
define wetting
the curving of the free surface of a liquid at the boundary line between a liquid, gas, and solid
what is wetting caused by?
different coefficients of the three states of matter
What must the net surface tension force be at points along the boundary line?
zero, which is possible if the free surface is curved
what is the contact angle?
the angle between the solid surface under the liquid and a tangent the the free surface, passing through a point on the boundary line
When is the liquid absolutely wetting?
when the contact angle is 0 degrees
When is the liquid wetting?
when the contact angle is from 0 to 90 degrees
When is the liquid non-wetting?
when the contact angle is between 90 and 180 degrees
When is the liquid absolutely non-wetting?
when the contact angle is 180 degrees
Why is wetting a relative property? and give examples
a liquid may wet some solid bodies but not others eg. water wets glass and not paraffin wax
What is the meniscus?
the curved free surface close to the walls of the vessel it’s in
When is the meniscus concave?
when the liquid wets the walls
When is the meniscus convex?
when the liquid doesn’t wet the walls
What is the Laplace pressure?
the pressure due to the unbalanced surface tension forces at the curved free surface
What does the Laplace pressure depend on? (Δp)
the radius of the curvature R of the meniscus
the alpha of the liquid
Δp = 2alpha/R
Where is the Laplace pressure directed?
towards the centre of the meniscus
Define capillary action.
rise or fall of the free surface of a liquid inside a small-diameter tube
What is a gas embolism?
when a gas bubble enters a capillary, it may stop the flow of blood
What are the possible complications of a gas embolism?
severe functional disorders and even death
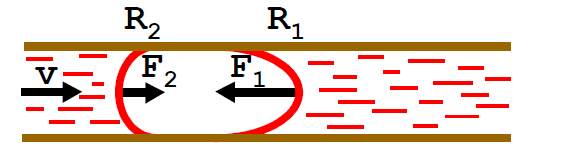
What happens to the blood in the capillary when there’s a gas embolism?
the front meniscus has a smaller radius
the Laplace pressure at the front meniscus is larger
this causes a net force opposing blood flow
Give examples of gas bubbles appearing in the bloodstream.
injecting liquids
surrounding pressure drops quickly
What is a similar phenomenon?
fat embolism caused by fat droplets in the bloodstream