Calculating moles of an element.
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
https://www.youtube.com/watch?v=-_-fNVmDwJk&list=PL9IouNCPbCxUhxxFUbR4SNfwmaRB8mYX3&index=7&ab_channel=Freesciencelessons
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
1
New cards
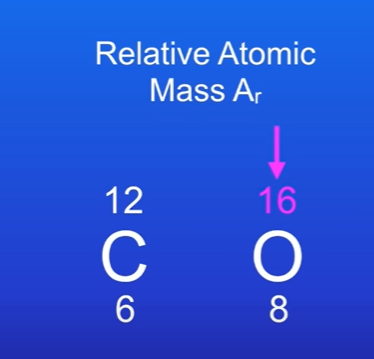
What number do we use for relative atomic mass. (Ar)
The top number of a element in periodic table.
2
New cards
How do we work out number of moles.
Number of moles = Mass (g) / Relative atomic mass (Ar)
3
New cards
Explain moles.
Carbon has Ar of 12, meaning 12g of carbon is 1 mole of carbon atoms.
Same for oxygen it has Ar of 16, meaning 16g of oxygen is one mole of oxygen atoms.
4
New cards
You are given a sample of magnesium with mass of 48g. How many moles of Mg have u been given
48 g / 24, which is 2 moles of magnesium
5
New cards