RP7 - Use of chromatography to investigate the pigments isolated from leaves of different plants, eg, leaves from shade-tolerant and shade-intolerant plants or leaves of different colours.
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
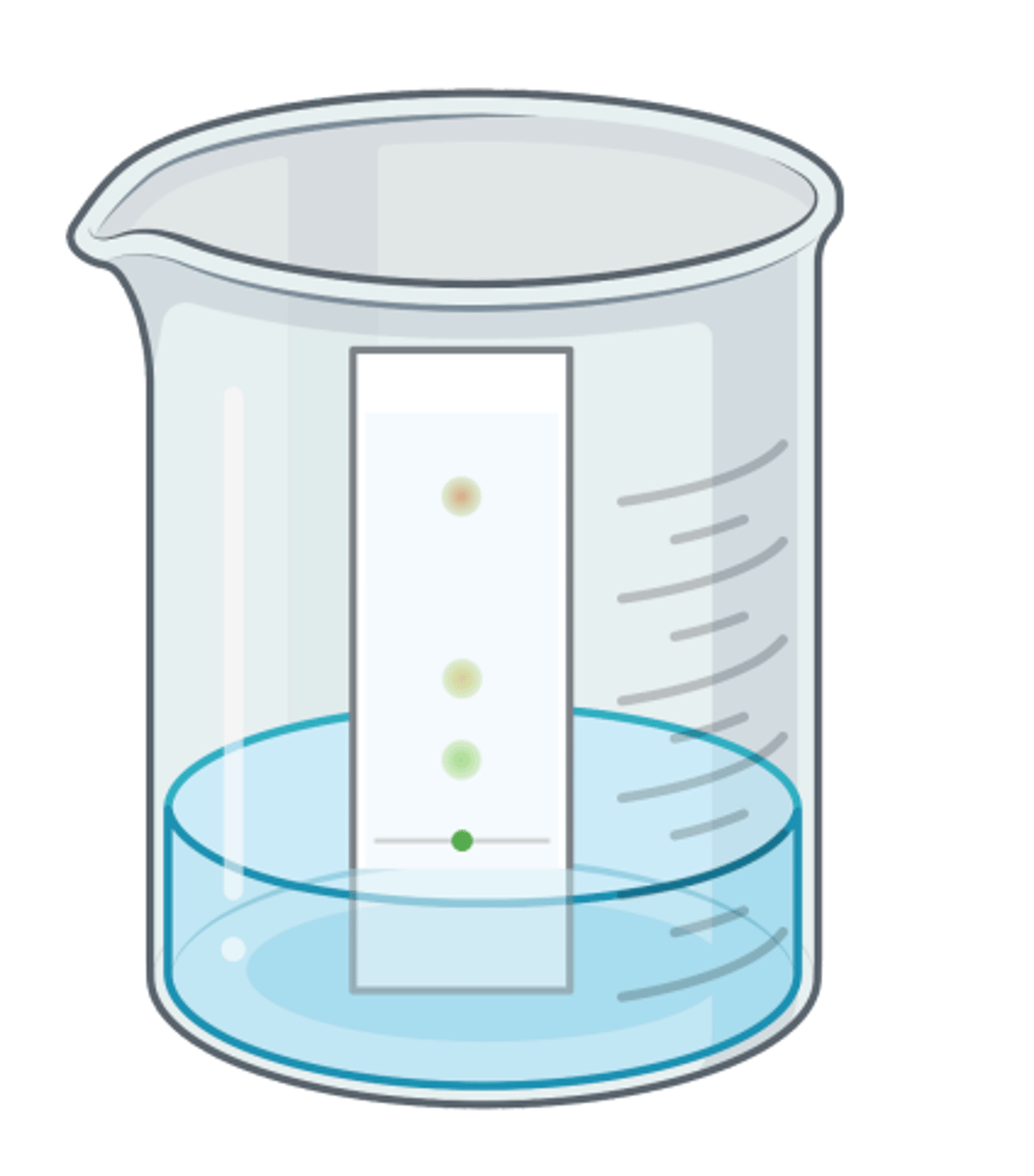
Describe how pigments from a leaf of a plant can be isolated with paper chromatography
1. Crush leaves with solvent to extract pigments
2. Draw a pencil line on chromatography paper, 1 cm above bottom
3. Add a drop of extract to line (point of origin)
4. Stand paper in beaker of (organic) solvent , ensuring solvent is below point of origin
5. Add lid and leave to run (solvent moves up, carrying dissolved pigments)
6. Remove before solvent reaches top and mark solvent front with pencil
Explain why the origin should be drawn in pencil rather than ink.
● Ink is soluble in solvent
● So ink would mix with pigments / line would move
Explain why the point of origin should be above the level of the solvent.
● Pigments are soluble in solvent
● So would run off paper / spots dissolve into solvent
Explain why a pigment may not move up the chromatography paper in one solvent
● May be soluble in one solvent but insoluble in another
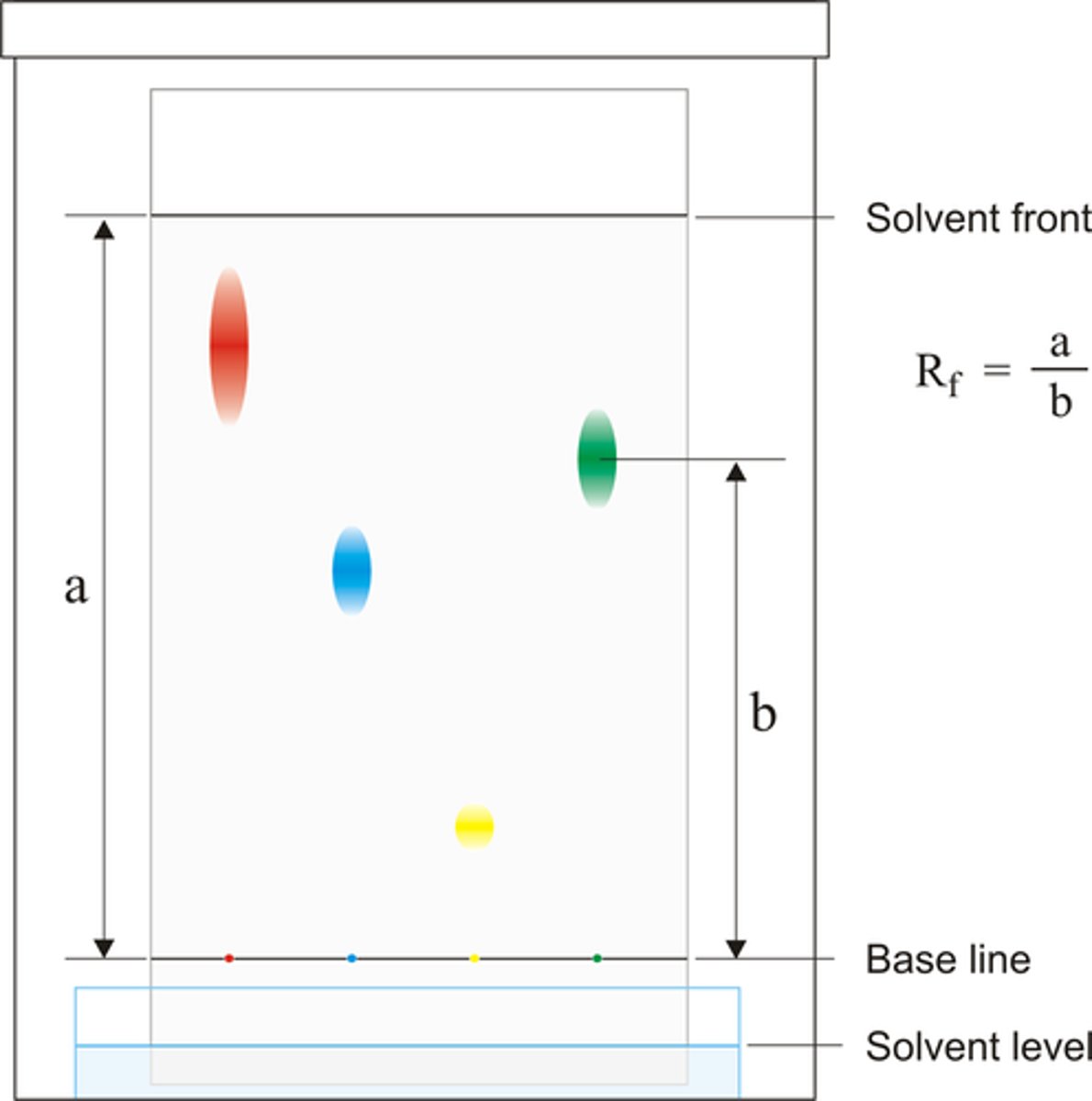
Describe how pigments can be identified
- Rf value = distance moved by spot / distance moved by solvent front
- Compare Rf value to published value
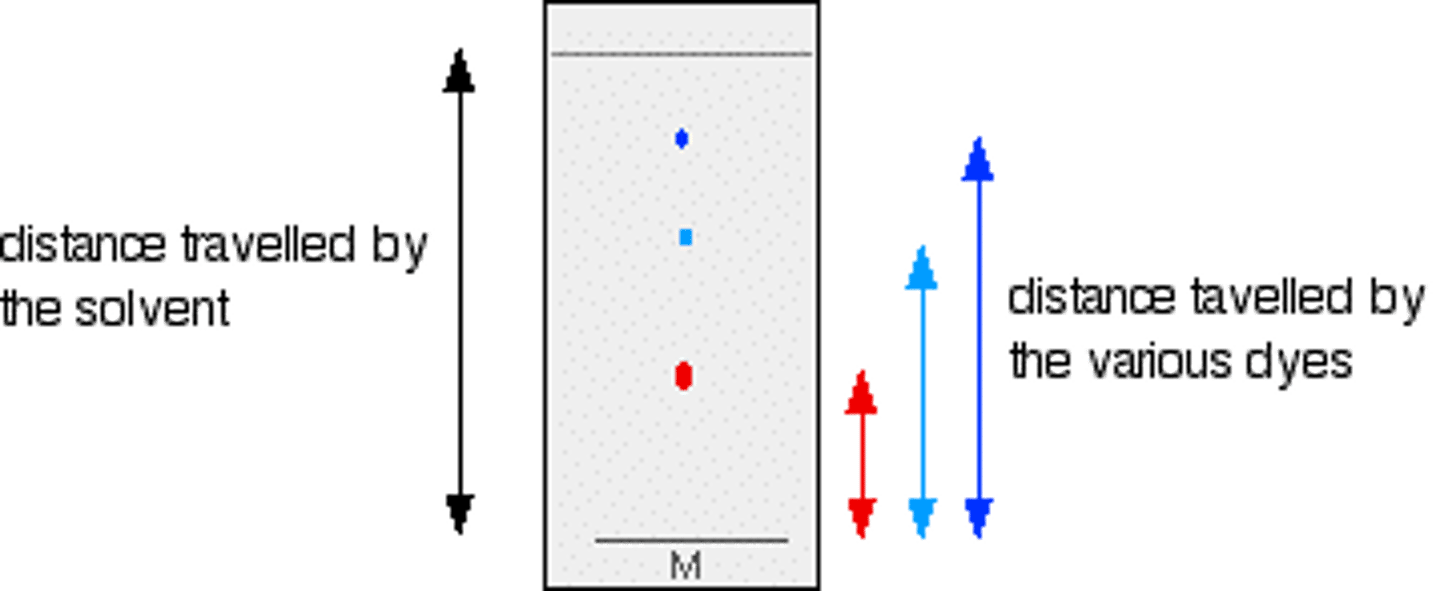
Explain why the solvent front should be marked quickly once chromatography paper is removed.
● Once solvent evaporates, solvent front not visible
Explain why the centre of each pigment spot should be measured.
● Standardises readings as pigment is spread out
● So allows comparisons to be made
Explain why the obtained Rf values were similar, but not identical, to the published values.
● May have used different solvent / paper / running conditions which would affect Rf value
Explain why Rf values are used and not the distances moved by pigment spots.
● The solvent and pigment may move different distances in different experiments, for example if paper was left in solvent for different time periods
● However the ratio ie. the Rf value will stay constant for same pigment
● so can be compared