Organic Chemistry 3 Exam
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
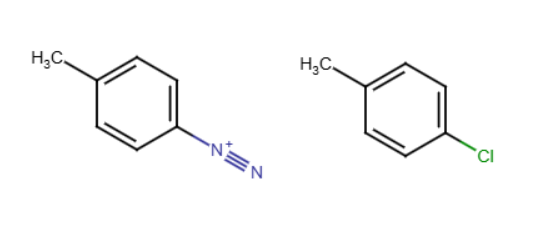
Sandmeyer reaction
When an aryl diazonium salt (triple bond between two nitrogen) reacts with CuCl or CuBr to form aryl chlorides or bromides
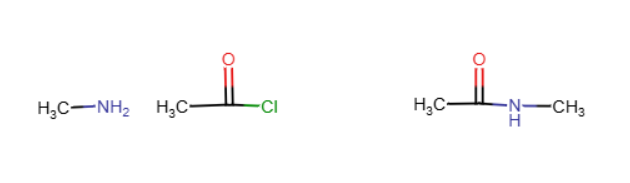
Amide synthesis
Typically two different products form, especially if pyridine is involved. Typically a NH2 replaces a Cl and the NH2 loses a hydrogen, making it NH. NaOH can be used instead of pyridine, having the same effect
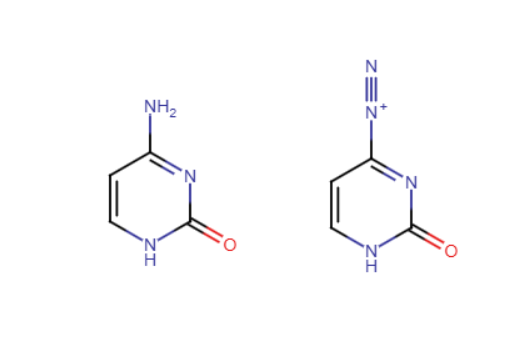
Diazotization
A nitrosonium ion (NaNO2/HCl) reacts with a 1° amine (NH) to form a diazonium salt (triple bond between two nitrogen)
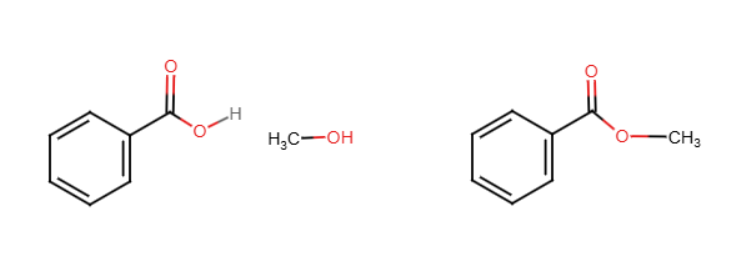
Fischer esterfication
Treatment of a carboxylic acid with an alcohol in the presence of an acid catalyst
Hydrolysis
Converting a molecule back to a carboxylic acid by adding excessive water
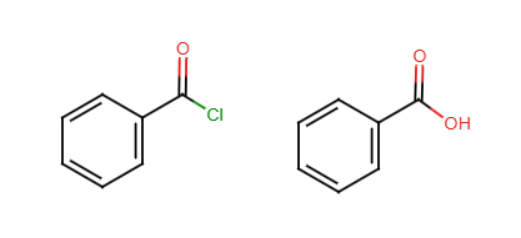
Acid chloride formation
A chloride replaces the OH, adding an O to the final product and creating HCl. Uses a reactant such as SOCl2. Products would be acid chloride, SO2, and HCl
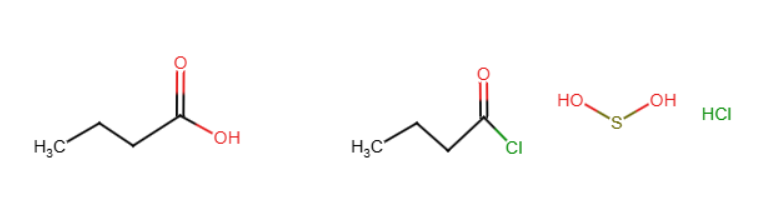
Hoffman elimination
I is used to add a group to the nitrogen, then Ag2O and heat are used to eliminate that group and form a double bond between two carbons that the group was attached to
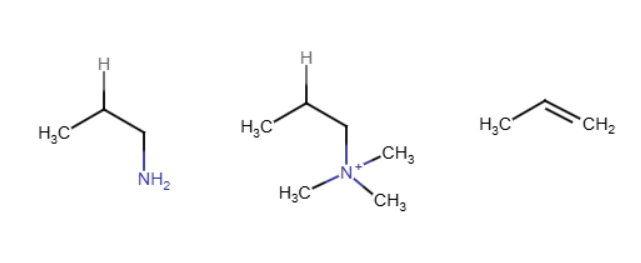
LiAlH4 in amines
Converts a nitrogen to NH2
KMnO4 and H2CrO4
Add carboxylic acids to molecules
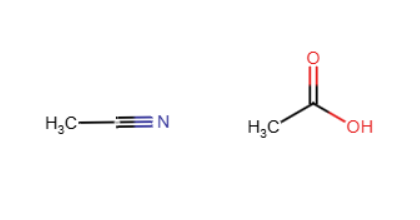
Hydrolysis of nitriles
A triple bond between C and N are replaced by 3 C-O bonds using H3O
LiAlH4
With carboxylic acids, it removes the oxygen that is double bonded, changing the C into CH2