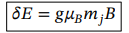Quantum mechanics term 1
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
Compton scattering equation
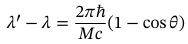
Equation for energy levels in an atom
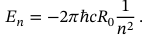
Postulates of Bohr’s model of the atom
-electrons move in fixed circular paths around the nucleus
-electrons in orbit do not radiate
Equation for electron angular momentum in Bohr’s model
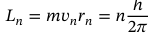
Equation for electron orbital radius in Bohr model
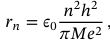
Heisenberg’s uncertainty principle

Schrodinger Equation
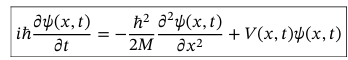
Probability of finding a particle in strip x —> x + dx
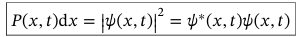
Normalisation condition for 1D system
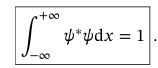
Time Independent Schr. Eq (TISE)
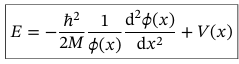
Solution to TISE
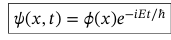
What effect does time dependence have on the probability of finding a particle in x—> x+dx and when is it significant?
-change of phase of wavefunction
-relevant when there are two contributions with different phases
Energy for each wavefunction describing a particle in an infinite potential well
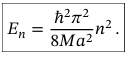
Conditions for a valid wavefunction
-function must be continuous and single valued for all positions and times
-integral of modulus squared of the function must be finite as to be normalised
-first derivative of wave function must be continuous everywhere except where potential has infinite step
Potential of mass-spring system
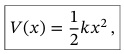
Energy for state n of the harmonic oscillator
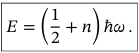
Zero Point energy
The lowest possible energy that a quantum mechanical system may have, which is not zero. In the case of a harmonic oscillator, it corresponds to the energy of the ground state.
Hermite Polynomials
A set of orthogonal polynomials that arise in the solution of the quantum harmonic oscillator, associated with the energy eigenstates.
Postulate 1 of quantum mechanics
For every dynamical system there is a wavefunction that is a continuous, square-integrable and single valued function of space and time.
Hamiltonian operator
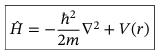
Momentum operator
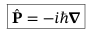
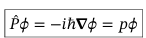
Eigenfunctions of the momentum operator
Plane waves with eigenvalues p = ℏk
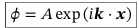
Hermitian operators
Operators that satisfy this equation
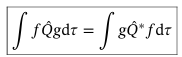
Postulate 2 of QM
Every dynamical variable is represented by a Hermitian Operator who’s eigenvalues represent possible results of measurements of a given variable
Postulate 3 of QM
Position and momentum operators are r and −𝑖ℏ∇. All other operators are the same as in classical physics
Orthonormality relation
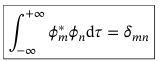
General wavefunction of the system
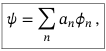
Coefficients in equation for general wavefunction of a system
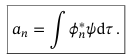
Eigenvalue equation for continuous variables
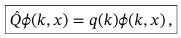
Orthonormality of eigenfunctions for continuous variables
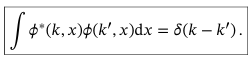
General wavefunction for continuous variables
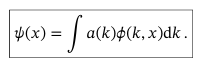
Coefficients a(k) in the equation for general wavefunction of continuous variables
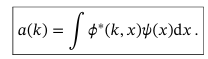
Expectation value
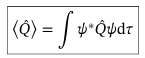
Commutator between x component of momentum operator and the position operator
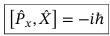
Commutators between i and j components of position operator and i and j components of momentum operator
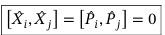
Commutator between i component of momentum operator and j component of position operator
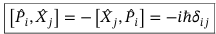
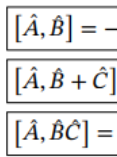
Commutator identities
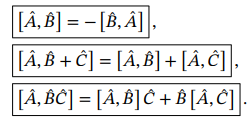
Generalised Heisenberg Uncertainty principle
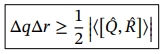
5th Postulate of QM
Between measurements, the development of the wavefunction with time is governed by the TISE
Time dependent wavefunction
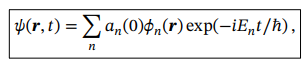
Degeneracy
two or more states with distinct wavefunctions which have the same eigenvalue of a dynamical variable
Ladder operators definition
operators that move us up and down the ladder of states where each step is a particular energy eigenstate
Angular momentum operator
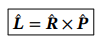
Operator for the square of the magnitude of angular momenum
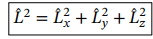
Angular momentum ladder operators

Schrodinger equation in 3D
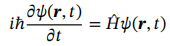
Hamiltonian operator in 3D
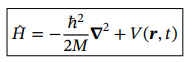
Potential energy operator in 3D
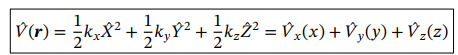
Potential function from a nucleus
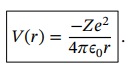
Condition between n (quantum number) and l (orbital angular momentum) for a hydrogen like atom
l < n
Energy levels in hydrogen like atoms in terms of energy of the ground state
E_{n}=-\frac{13.6Z^2}{n^2}eV
Change in energy of state when an energy level with fixed n and l splits due to the value of magnetic quantum number, m
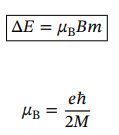
Adding two angular momenta possibilities
each result has a distinct probability
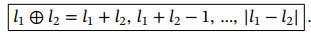
Pauli Matrices for fermions
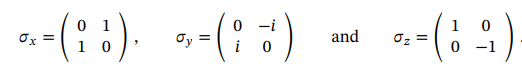
Spin ½ operators in terms of Pauli matrices
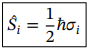
total angular momentum
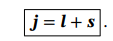
Spin orbit coupling operator
f\left(r\right)=\frac{\mu_0Ze^2}{4\pi M^2r^3}
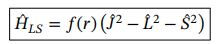
Hamiltonian operator of the effect of an external magnetic field
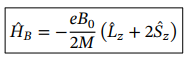
Correction to energy states, including both external magnetic field and spin-orbit coupling
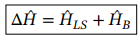
Correction to energy for strong field zeeman effect
ml and ms eigenvalues of operators Lz and Sz
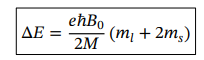
Correction to energy for weak field Zeeman effect