Acids, Alkali and Titrations
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
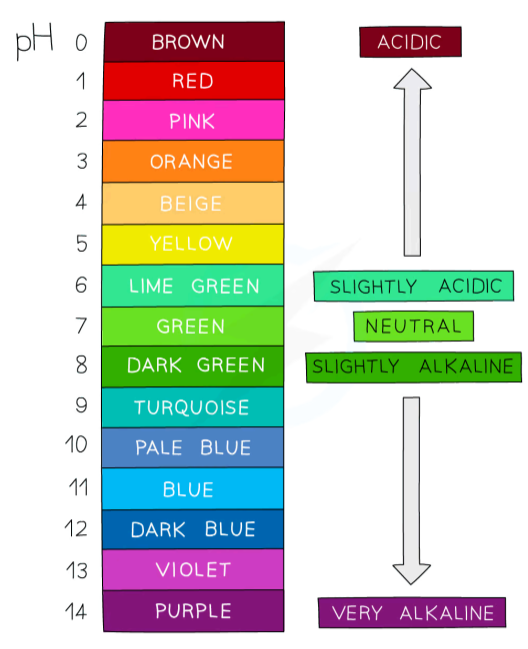
pH scale?
from 0-14
acids- 0 to 7
alkali- 7 and above
pH 0-3= strong acid
pH 4-6= weak acid
pH 8-10= weak alkali
pH 11-14= strong alkali
pH 7 is neutral (water)
Litmus colour in acid and alkali?
acid- red
alkali- blue
Phenolphthalein colour in acid and alkali?
acid- colourless
alkali- pink
Methyl orange colour in acid and alkali?
acid- red
alkali- yellow
acids and alkalis in water form?
acids forms a H+ ion- makes a solution acidic
alkalis forms a OH- ion- makes aqueous solution an alkali
Neutralisation reaction?
occurs when an acid reacts with an alkali
the H+ and OH- ion produces water
Acid + Base = H2O + salt
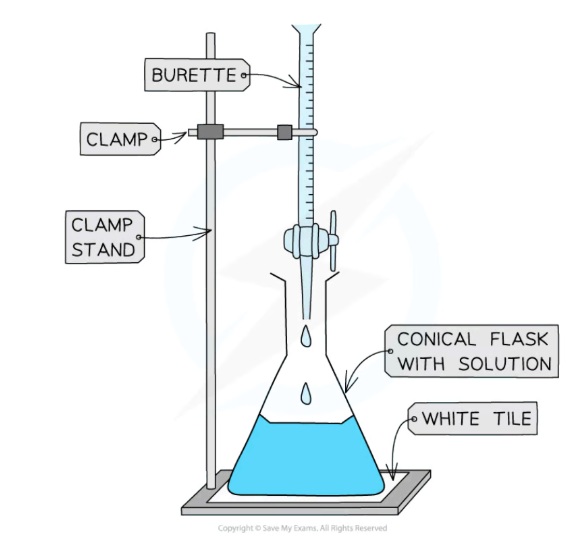
Titrations?
A method of analysing the concentration of solutions
Method of doing a titration
Use a pipette and a pipette filler and place 25cm³ of sodium hydroxide solution into a conical flask.
Fill the burette with hydrochloric acid (do this below eye level)
Record the starting point on the burette to the nearest 0.05 cm³
Place the conical flask on a white tile and add a few drops of phenolphthalein indicator to the conical flask.
Tip of the burette should be inside the conical flask
Open the tap of the burette and let the acid flow through, keep vigorously swirling the conical flask.
Swirl slowly when you think the end-point (colour change) is about to be reached.
The indicator changes colour when all the alkali has been neutralised (phenolphthalein will turn pink)